��Ŀ����
A��B��C��D��E����5��Ԫ�ء�����գ�
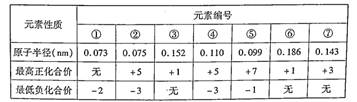
��1��AԪ�ػ�̬ԭ�ӵ��������3��δ�ɶԵ��ӣ��������2�����ӣ���Ԫ�ط���Ϊ_______��
��2��BԪ�صĸ�һ�����ӵĵ��Ӳ�ṹ�����ͬ��CԪ�ص���һ�����ӵĵ��Ӳ�ṹ�����ͬ��B��C�γɵĻ�����Ļ�ѧʽΪ________���侧����ÿ��B������Χ��_____��C������֮�����ڡ��侧�����Ҫ���������У�___________________________________(д����������)��
��3��DԪ�ص����������ӵ�3d�Dz�Ϊ�������D��Ԫ�ط���Ϊ______�����̬ԭ�ӵĵ�
���Ų�ʽΪ___________________________________��
��4��EԪ�ػ�̬ԭ�ӵ�M��ȫ������N��û�гɶԵ��ӣ�ֻ��һ��δ�ɶԵ��ӣ��䵥����һ��������������(S)��Ӧ����д���÷�Ӧ����ʽ _____________________________��
��1��AԪ�ػ�̬ԭ�ӵ��������3��δ�ɶԵ��ӣ��������2�����ӣ���Ԫ�ط���Ϊ_______��
��2��BԪ�صĸ�һ�����ӵĵ��Ӳ�ṹ�����ͬ��CԪ�ص���һ�����ӵĵ��Ӳ�ṹ�����ͬ��B��C�γɵĻ�����Ļ�ѧʽΪ________���侧����ÿ��B������Χ��_____��C������֮�����ڡ��侧�����Ҫ���������У�___________________________________(д����������)��
��3��DԪ�ص����������ӵ�3d�Dz�Ϊ�������D��Ԫ�ط���Ϊ______�����̬ԭ�ӵĵ�
���Ų�ʽΪ___________________________________��
��4��EԪ�ػ�̬ԭ�ӵ�M��ȫ������N��û�гɶԵ��ӣ�ֻ��һ��δ�ɶԵ��ӣ��䵥����һ��������������(S)��Ӧ����д���÷�Ӧ����ʽ _____________________________��
��1�� N ��1�֣�
��2��CsCl��1�֣���8��1�֣�������ʱ�ܵ��硢�ϸߵ��۵�ȣ�1�֣�
��3��Fe��1�֣���1S22S22P63S23P63d64S2��[Ar] 3d64S2��1�֣�
��4��2Cu + S
 Cu2S��2�֣�д��Cu��δд����ȷ����ʽ��1�֣�
Cu2S��2�֣�д��Cu��δд����ȷ����ʽ��1�֣������������1���������3��δ�ɶԵ��ӣ��������2�����ӣ�˵��2P�������3�����ӣ�AΪN��
��2��BԪ�صĸ�һ�����ӵĵ��Ӳ�ṹ�����ͬ��˵��BΪCl��CԪ�ص���һ�����ӵĵ��Ӳ�ṹ�����ͬ��˵��CΪCs�������γɵĻ�����ΪCsCl���侧��ṹΪ���������ṹ��ÿ��Cl?��Χ��8��Cs+��֮��������CsCl����Ϊ���Ӿ��壬��������ʱ�ܵ��硢�ϸߵ��۵�����ʡ�
��3��DԪ�ص����������ӵ�3d�Dz�Ϊ���������Dԭ��3d����6�����ӣ�DԪ��ΪFe��
��4��EԪ�ػ�̬ԭ�ӵ�M��ȫ������N��û�гɶԵ��ӣ�ֻ��һ��δ�ɶԵ��ӣ�˵��3d����Ϊ10�����ӣ�N��û�гɶԵ��ӣ�ֻ��һ��δ�ɶԵ��ӣ�4S����1�����ӣ�MΪCu��Cu��S���ȷ�Ӧ����Cu2S��

��ϰ��ϵ�д�
�����Ŀ

 YZ- (aq)+X3W+(aq) ��H="+45.6" KJ/mol
YZ- (aq)+X3W+(aq) ��H="+45.6" KJ/mol