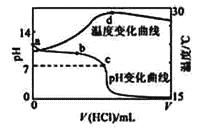
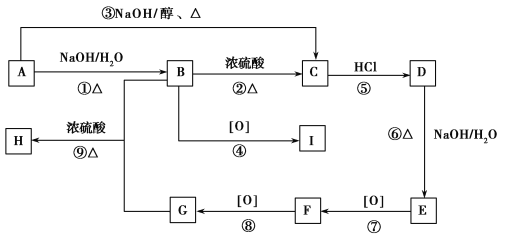
��Ŀ����
����Ŀ��������Ԫ��A��B��C��D��AԪ�ص�ԭ�����������Ų�ʽΪms1��BԪ�ص�ԭ�Ӽ۵����Ų�ʽΪns2np2��CԪ��λ�ڵڶ�������ԭ����p�ܼ�������s�ܼ��������������DԪ��ԭ�ӵ�L���p�ܼ�����3��δ�ɶԵ��ӡ�
(1)CԪ��ԭ�ӻ�̬ʱ�ļ۵����Ų�ʽΪ________����AԪ��Ϊ�ǽ���Ԫ����A��C�γɵĻ������еĹ��ۼ�����________��(��ҡ��С�)��
(2)��n��2ʱ��B�������̬�⻯��ķ��ӹ���Ϊ________������ԭ�ӵ��ӻ���ʽΪ________��BC2����________����(����ԡ��Ǽ��ԡ�)����n��3ʱ��B��C�γɵľ�������________���塣
(3)��AԪ�ص�ԭ�����������Ų�ʽΪ2s1��BԪ�ص�ԭ�Ӽ۵����Ų�ʽΪ3s23p2��A��B��C��D����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ____________________(��Ԫ�ط���)��
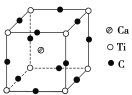
(4)��ͼΪCԪ�����ѡ���Ԫ���γɵ�ij����ṹ�е���С�ظ���Ԫ���þ�����ÿ����ԭ����Χ��������Ҿ�����ȵĸ�������________�����þ���Ļ�ѧʽΪ________________________��
���𰸡�(1)2s22p4 �� (2)�������� sp3 �Ǽ��� ԭ��
(3)N��O��Si��Li (4)8 CaTiO3
��������������Ԫ��A��B��C��D��AԪ�ص�ԭ�����������Ų�ʽΪms1����Aλ����A����BԪ�ص�ԭ�Ӽ۵����Ų�ʽΪns2np2��B������A����CԪ��λ�ڵڶ�������ԭ����p�ܼ�������s�ܼ����������������ԭ�Ӻ�������Ų�ʽΪ1s22s22p4����CΪ��Ԫ�أ�DԪ��ԭ�ӵ�L���p�ܼ�����3��δ�ɶԵ�������DԪ��ԭ�ӵ����Ų�ʽΪ1s22s22p3����DΪ��Ԫ�ء�
(1)Aλ����A������AԪ��Ϊ�ǽ���Ԫ������AΪ��Ԫ��������Ԫ�صĻ�����ΪH2O��H2O2���������еĹ��ۼ�Ϊ�Ҽ���
(2)��n��2ʱ��BΪ̼Ԫ�����������̬�⻯�����ʽΪCH4��Ϊ��������ṹ��������ԭ���ӻ���ʽΪsp3�ӻ���BC2ΪCO2��CO2Ϊֱ���νṹ��������������������غ������ڷǼ��Է�������n��3ʱ��BΪSiԪ��������Ԫ���γɵĻ�����ΪSiO2������ԭ�Ӿ��塣
(3)��AԪ�ص�ԭ�����������Ų�ʽΪ2s1��AΪ�Ԫ����BԪ�ص�ԭ�Ӽ۵����Ų�ʽΪ3s23p2��BΪ��Ԫ����ͬ����Ԫ�ص�һ�����ܴ������������ϳ������������ǽ�����Խǿ���һ������Խ������NԪ��ԭ��2p�ܼ�����3��������Ϊ�����״̬����NԪ�صĵ�һ�����ܸ���OԪ����������Ԫ�ص�һ�����ܴ�С˳��Ϊ��N��O��Si��Li��
(4)�ɾ���ͼ��֪��ÿ����ԭ����Χ��������Ҿ�����ȵĸ�����λ��������ÿ������Ϊ8������������������Tiԭ������Ҿ�����ȵĸ�������8�������ݾ�̯����֪�����ڶ��ǵ�ԭ��ͬʱΪ8��������������ÿ��ԭ����1/8���ڸþ������������ϵ�ԭ����ͬʱ��4��������������ÿ��ԭ����1/4���ڸþ�������������λ�õ�ԭ������ȫ���ڸþ���������N(Ca)��N(Ti)��N(O)��1��![]() ��
��![]() ��1��1��3���þ���Ļ�ѧʽΪCaTiO3��
��1��1��3���þ���Ļ�ѧʽΪCaTiO3��