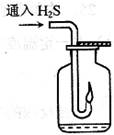
��Ŀ����
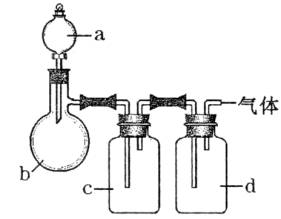
������ͼ��ʾװ����ȡ���е����ָ�����������壨ͼ������̨�����С����ȼ������ռ�װ�þ�����ȥ����Ҫʱ���Լ��ȣ�a��b��c��d��ʾ��Ӧ�����м�����Լ�����
��1�����������п��Եõ����������������____________________��
��2��ָ������������������ȡ�����壬��˵�����ɡ�
������__________��������____________________��
������__________��������____________________��
������__________��������____________________��

| ���� | a | b | c | d |
| C2H4 | �Ҵ� | ŨH2SO4 | NaOH��Һ | ŨH2SO4 |
| Cl2 | Ũ���� | MnO2 | NaOH��Һ | ŨH2SO4 |
| NH3 | ����NH4Cl��Һ | ��ʯ�� | H2O | ����NaOH |
| NO | ϡHNO3 | ͭм | H2O | P2O5 |
��2��ָ������������������ȡ�����壬��˵�����ɡ�
������__________��������____________________��
������__________��������____________________��
������__________��������____________________��
��1��NO ��2����C2H4 װ�������¶ȼƣ������ƻ�ѧ��Ӧ�¶� ��Cl2 ��Ӧ���ɵ�Cl2��c�е�NaOH��Һ������ ��NH3��Ӧ���ɵ�NH3��c�е�H2O������
���������Ʊ�װ����û���¶ȼƶ��������¶ȹ����Ƶø��﴿����C2H4������װ�ú��Լ���Ȼ�����Ƶ�Cl2����cװ���е�NaOH��Һ��ϴ��ʱ���Ƶõ�Cl2ȫ�����գ���ܵõ�Cl2������NH4Cl��Һ��Ca��OH��2��������NH3����NH3ͨ��cװ��ϴ��ʱȫ����ˮ���գ����Ҳ���ܵõ�NH3��Cuм��ϡHNO3��Ӧ���Ʊ�NO����Ȼ�в��ֱ�װ����O2����������ͨ��c��ˮϴ�����ɵIJ���NO2ȫ��ת����NO����P2O5�����ɵô�����NO��

��ϰ��ϵ�д�
 Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
�����Ŀ
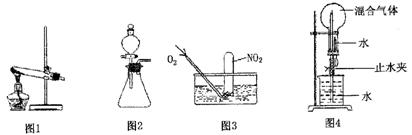

 �������������ʵ�����Ʒ����������������ա�
�������������ʵ�����Ʒ����������������ա�