��Ŀ����
����Ŀ�����ܱ������з�����ӦX(g)��3Y(g)![]() 2Z(g)����X��Y��Z����ʼŨ�ȷֱ�Ϊ0.1mol��L��1��0.3mol��L��1��0.2mol��L��1����һ�������£�����Ӧ�ﵽһ����ʱ�������ʵ�Ũ�ȿ����ǣ� ��
2Z(g)����X��Y��Z����ʼŨ�ȷֱ�Ϊ0.1mol��L��1��0.3mol��L��1��0.2mol��L��1����һ�������£�����Ӧ�ﵽһ����ʱ�������ʵ�Ũ�ȿ����ǣ� ��
A. XΪ0.2 mol��L��1B. YΪ0.1 mol��L��1
C. ZΪ0.4 mol��L��1D. ZΪ0.1 mol��L��1ʱ��YΪ0.4 mol��L��1
���𰸡�B
��������
����Ӧ������Ӧ������У����ɵ�Z��Ũ��С��0.2 mol��L -1 ����ƽ��ʱZ��Ũ��С��0.4 mol��L -1 ������Ӧ���淴Ӧ�����ƶ������ɵ�X2 ��Y2 ��Ũ��С��0.1 mol��L -1��0.3 mol��L -1����ƽ��ʱX2��Ũ��С��0.2 mol��L -1 ��Y2 ��Ũ��С��0.6 mol��L -1 ��
A. ƽ��ʱX2��Ũ��С��0.2 mol��L��1��ѡ��A����
B. ƽ��ʱY��Ũ��С��0.6 mol��L -1������0������Ϊ0.1 mol��L��1��ѡ��B��ȷ��
C. ƽ��ʱZ��Ũ��С��0.4 mol��L��1 ��ѡ��C����
D. ZΪ0.1 mol��L��1ʱ��YΪ0.45mol��L��1��ѡ��D����
��ѡB��

 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�����Ŀ��ijʵ��С���ռ���ͭ��Ũ���ᷴӦ�����ɵ��������̽�����Իش���������
(1)��ͼ����ͭ��Ũ���ᷴӦ����ʵ��װ��
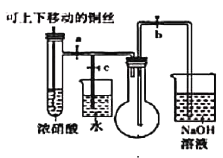
��ָ��װ���еĴ���_________________________��
��װ�øĽ���Ϊ��ʹ���ɵ����������ƿ��ʵ��ʱ�ȹرյ��ɼ�_______���ٴ��ɼ�________��
�۵����������ƿ��___________________________������ʹ��Ӧֹͣ��
(2)����ƿ�г�������ɫ������ʱֹͣʵ�飬ȡ����ƿ��������Ƥ����
�ٰ���ƿ������װˮ��ˮ���в�����ƿ����Ƥ������ƿ��ˮ�����������ݻ�ѧ����ʽ3NO2+H2O=2HNO3+NO������ƿ����Һ��Ũ����______mol/L(��������δ��ɢ��ˮ�ۣ��ҵ�ʱ�����������Ħ�����Ϊ25L/mol)
�ڰ���ƿ������װˮ��ˮ���в�����ƿ����Ƥ����ʵ��ʱ������ƿ��Һ����������������������ƿ���������������ԭ��������ռ���NO2�к���__________��
A.NO B.N2O4 C.O2 D.����
��С��ͬѧ�������Ϸ��֣�NO2����ˮʱ����������������2NO2+H2O=HNO3+HNO2��Ϊ��֤���������ʣ�������ٵ���Ҫԭ��С��ͬѧ����ƿ�е���Һ�μӸ��������Һ�����ָ��������Һ��ɫ��д�����������Һ��ɫ�����ӷ���ʽ____________________________��
(3)HNO2��һ�ֲ��ȶ��ֽ�������.2HNO2=NO��+NO2��+H2O����.3HNO2=HNO3+2NO��+H2O��Ϊ��̽����ͬ�¶ȡ�Ũ����������ķֽ���С��ͬѧ��������ʵ�飺
��һ��������1mol/LNaNO2��Һ100mL(��Ϊ��ҺA)��
�ڶ�����ȡ��ҺA�ֱ��ˮϡ��Ϊ0.5mol/L��0.2mol/L��0.1mol/L����Һ�����α�Ϊ��ҺB��C��D��
���������ֱ�ȡ���ι���ҺA~D��Һ���Թ��У�������Ƥ�������ڲ�ͬ�¶ȵ�ˮ�м���5min���ټ���һ�ι�10mol/LH2SO4��Һ������������Ƥ�����۲������������ɫ
ˮԡ�¶�/�� | A | B | C | D |
��ˮԡ | ����ɫ | ����ɫ | ����ɫ | ����ɫ |
80-70 | ����ɫ | ����ɫ | ����ɫ | ��ɫ |
50-40 | ����ɫ | ��Һ���Ϻ���ɫ | ��ɫ | ��ɫ |
20-10 | ��Һ���Ϻ���ɫ | ��ɫ | ��ɫ | td style="width:88.95pt; border-top-style:solid; border-top-width:0.75pt; border-left-style:solid; border-left-width:0.75pt; padding:3.38pt 5.03pt; vertical-align:middle">
�ٵ�һ��ʵ��Ҫ�õ����������У��ձ���100mL����ƿ����������___________��
����NaNO2��Һ���������ֱ����HNO2��Һ��ԭ����______________��
��ͨ��ʵ������ó����ۣ�____________�����£������ᰴ��Ӧ�����ֽ⡣