��Ŀ����
����Ŀ��
����ֲ���纣���������к��зḻ�ĵ�Ԫ�أ���Ԫ���Ե����ӵ���ʽ���ڡ�ʵ���ҴӺ�������ȡ����������£�
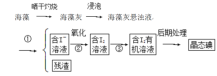
��1��д����ȡ��Ĺ������й�ʵ����������ƣ���______����________��
��2����ȡ��Ĺ����пɹ�ѡ����л��ܼ���
A�����͡��ƾ�
B�����Ȼ�̼������
C�����ᡢ�ƾ�
��3��Ϊ�����������������������ʵ���������ձ���������������̨����ƿ�����ܡ��ƾ��ƣ���ȱ�ٵIJ���������____________________________________��
��4���Ӻ�����л��ܼ�����ȡ��ͻ����л��ܼ�������Ҫ��������ָ����ͼ��ʾ��ʵ��װ���еĴ���֮������ ���� ���� ���� ��
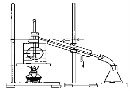
��5��Ϊ���ڿ�������ʱ���¶ȣ�����ʱʹ��ˮԡ���ȣ���������________��ۼ���
���𰸡�
��1������������ȡ
��2��B
��3��©������Һ©��
��4�����¶ȼƲ����λ�ô�������ȴˮ�����������ȱ�ٷ�ʯ��ȱ��ʯ����
��5��������ƿ
��������
�����������1������������Һ���ù��ˣ�������ˮ�еĵⵥ����ȡ������ѡ����ʵ���ȡ�����ɣ�
��2����ȡ�Ļ���ԭ�������ܼ��������ܣ���������һ���ܼ��е��ܽ�ȱ�����һ�ִ�Ķ࣬�ʿ������Ȼ�̼���������ƾ���������ˮ�ǻ��ܵģ�����ѡ�ʴ�ΪB��
��3������ʱ��Ҫ©�����ձ�����������ʹ������е�����ת��Ϊ����л���Һ���������ȡ��Һ����ȡ��Һ�����õ���Һ©�������Գ�������֪�����⣬ȱ�ٷ�Һ©����©����
��4������ˮӦ���½��ϳ����ձ�����Ӧ��ʯ�������¶ȼ�ˮ����Ӧ����ƿ֧�ܿ���ƽ��
��5��ˮԡ���ȵ��¶���ʹ�Թ��ڻ��ձ����Լ������¶Ⱦ��ȣ����г�ʱ������¶ȱ��ֺ㶨���ص㣮���Ȼ�̼�е㣺76.8�����ⵥ�ʷе�Ϊ184���������̬����������ƿ��ۼ���

 ������״Ԫ��ҵϵ�д�
������״Ԫ��ҵϵ�д� ��ʱ�ƿ�������ϰϵ�д�
��ʱ�ƿ�������ϰϵ�д� һ��һ��һ��ͨϵ�д�
һ��һ��һ��ͨϵ�д�����Ŀ����ѧ�����������Ź㷺��Ӧ�á����ж�Ӧ��ϵ������ǣ� ��
ѡ�� | ��ѧ���� | ʵ��Ӧ�� |
A | Al2(SO4)3��С�մ�Ӧ | ��ĭ�������� |
B | ����ͭ������ǿ | FeCl3��ʴCu����ӡˢ��·�� |
C | �������ξ��������� | Ư��Ư��֯�� |
D | ����������� | ������ȥ��ˮ�� |
A. A B. B C. C D. D


