��Ŀ����
19����ˮռ�����ܴ�ˮ����97.2%�����Ѻ�ˮ�����ͻ�����������������ȿ��Խ����ˮ��Դȱ�������⣬�ֿ��Գ�����ú�����Դ����1��Ŀǰ������ʵ�õġ���ˮ��������Ҫ����֮һ�����������������仯���������仯����ѧ�仯����
��2����ˮɹ�η���ʳ�ξ�����ĸҺ�к���KCl��MgCl2���������롢�ᴿ�����ڹ�ҵ��ұ�������غ�þ��
��3����ҵ�����õ�⣨��������Ϊʯī������ʳ��ˮ���Ƶ���Ҫ������Ʒ����Ӧ�����ӷ���ʽΪ2Cl-+2H2O$\frac{\underline{\;���\;}}{\;}$Cl2��+H2��+2OH-��������õ�ʳ��ˮ��Ҫ���ƣ�ԭ���Ǵ����к�����ɳ��Ca2+��Mg2+��SO42-�����ʣ��ڼ�����Һ�л��γɳ����������ӽ���Ĥ�����õ������������36.5%��Ũ����1000t��������Ҫ����ʳ��585t��
��4�����������������һ�������ȼҵ��Ʒ���Ȼ���ѭ������������������������ն�������ķ������÷������������£�
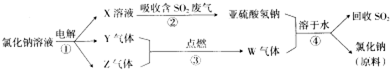
��д���ڢܵĻ�ѧ��Ӧ����ʽ��
��SO2+NaOH=NaHSO3����NaHSO3+HCl=NaCl+SO2��+H2O��
���� ��1������ֻ��ˮ��״̬��Һ̬����̬�ٵ�Һ̬�Ĺ��̣��������������仯��
��2��ʳ�ε��ܽ�����¶�Ӱ��仯�������ú�ˮɹ�Σ�����ʳ�ξ�����ĸҺ�к���KCl��MgCl2���������롢�ᴿ�����ڹ�ҵ��ұ�������غ�þ��
��3����ⱥ��ʳ��ˮʱ�������������ӷŵ磬�����������ӷŵ磬ͬʱ��Һ�������������ƣ������к�����ɳ��Ca2+��Mg2+��SO42-�����ʣ��ڼ�����Һ�л��γɳ����������ӽ�������ⱥ��ʳ��ˮ�ķ���ʽΪ2NaCl+2H2O$\frac{\underline{\;���\;}}{\;}$2NaOH+Cl2��+H2�������ݻ�ѧ����ʽ���㣻
��4����������ͼ����������Ȼ�����Һ�õ�����������������������Һ������۷�Ӧ����Ϊ��ȼ��Ϊ������������Ӧ����WΪHCl�������X��Һ�Ͷ�������Ӧ�������������ƣ�XΪNaOH���������������������Ʒ�Ӧ�����Ȼ��ơ����������ˮ�������ԭ��ΪNaCl����������NaCl���ɣ�ͬʱ���ն������ڹ����Ķ���������������Ʒ�Ӧ����NaHSO3���ܸ����������ʽ������ǿ�ᷴӦ������
��� �⣺��1����������ֻ��ˮ��״̬��Һ̬����̬�ٵ�Һ̬�Ĺ��̣��������������仯��
�ʴ�Ϊ�������仯��
��2��ʳ�ε��ܽ�����¶ȵ�Ӱ��С����������ͷ�����ˮ�֣�ʹ�Ȼ��Ƴɾ�������������ʳ�ξ�����ĸҺ�к���KCl��MgCl2���������롢�ᴿ���õ�ⷨұ�������غ�þ��
�ʴ�Ϊ��KCl��MgCl2����ҵ��ұ�������غ�þ��
��3����ⱥ��ʳ��ˮʱ�������������ӷŵ磬�����������ӷŵ磬ͬʱ��Һ�������������ƣ����ӷ���ʽΪ��2Cl-+2H2O$\frac{\underline{\;���\;}}{\;}$Cl2��+H2��+2OH-�������к�����ɳ��Ca2+��Mg2+��SO42-�����ʣ��ڼ�����Һ�л��γɳ����������ӽ�������ⱥ��ʳ��ˮ�ķ���ʽΪ2NaCl+2H2O$\frac{\underline{\;���\;}}{\;}$2NaOH+Cl2��+H2�������跴Ӧ����������ģ����ɵ�����������ȫ�������Ȼ��⣬��������Ԫ���غ�NaCl��HCl��ʳ�ε�����=$\frac{1000t��36.5%}{36.5}$��58.5t=585t��
������Ҫ����ʳ��585t��
�ʴ�Ϊ��2Cl-+2H2O$\frac{\underline{\;���\;}}{\;}$Cl2��+H2��+2OH-�������к�����ɳ��Ca2+��Mg2+��SO42-�����ʣ��ڼ�����Һ�л��γɳ����������ӽ���Ĥ��585��
��4������٣���ⱥ��ʳ��ˮ�ķ���ʽΪ2NaCl+2H2O$\frac{\underline{\;���\;}}{\;}$2NaOH+Cl2��+H2���������X��Һ�Ͷ�������Ӧ�������������ƣ�XΪNaOH������۷�Ӧ����Ϊ��ȼ��Ϊ������������Ӧ����WΪHCl�����Ԣ�Ϊ������������������������Ʒ�Ӧ�����������ƺ�ˮ����ѧ����ʽΪ��SO2+NaOH=NaHSO3��������������ʽ��NaHSO3��ǿ��HCl��Ӧ��NaHSO3+HCl=NaCl+SO2��+H2O��
�ʴ�Ϊ��SO2+NaOH=NaHSO3��NaHSO3+HCl=NaCl+SO2��+H2O��
���� �����Ժ�ˮ�����ͻ�������Ϊ���������������ʵķ��롢�Ʊ��Լ�����ʽ����д��֪ʶ��������ѧ������֪ʶ����������������Ŀ�Ѷ��еȣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д���1�����öԱ������ӵ�ؿɼ�ⰱ�������ط�Ӧԭ��Ϊ2NH3+
 ���Ա������TN2H4+
���Ա������TN2H4+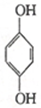 ���Ա��ӣ���N2H4�ĽṹʽΪ
���Ա��ӣ���N2H4�ĽṹʽΪ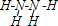 ���õ�������ĵ缫��ӦʽΪ
���õ�������ĵ缫��ӦʽΪ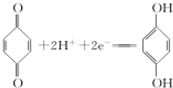 ��
����2��25��ʱ����nmol•L-1�İ�ˮ��0.1mol•L-1������������ϣ�
���������Һ��c��NH4+��=c��Cl-��������Һ��pH=7��
���������Һ��c��NH4+����c��Cl-������Ӧ���������Ϊa��
a������㣬��ˮʣ�� b����ˮ������ǡ�÷�Ӧ c���������
��3����0.5L�����ܱ������У�һ������N2��H2���з�Ӧ��N2��g��+3N2��g��?2NH3��g����H=bkJ•mol-1���仯ѧƽ�ⳣ��K���¶ȵĹ�ϵ���£�
| �¶�/�� | 200 | 300 | 400 |
| K | 1.0 | 0.86 | 0.5 |
��400��ʱ�����ijʱ�̰��������������������ʵ����ֱ�Ϊ3mol��2mol��1molʱ����ʱ�̸÷�Ӧ��u����N2��С�ڣ�����ڡ���С�ڡ����ڡ���u����N2����
��4����֪����4NH3��g��+3O2��g���T2N2��g��+6H2O��g����H=-1266.8kJ•mol-1
��N2��g��+O2��g���T2NO��g����H=+180.5kJ•mol-1
д�������´��������Ȼ�ѧ����ʽ��4NH3��g��+5O2��g��=4NO��g��+6H2O��g����H=-905.8KJ/mol��
| A�� |  | B�� |  | C�� |  | D�� |  |
| A�� |  ����ͼװ�ñȽ�Na2CO3��NaHCO3���ȶ��� | |
| B�� |  ����ͼװ������NH3������ֹ���� | |
| C�� |  ����ͼװ������AlCl3������Һ�Ʊ�AlCl3���� | |
| D�� |  ����ͼװ����ȡFe��OH��2���� |

| A�� | ͼI��ʾ����μӵ�0.1mol/Lij����Һ�еõ��ĵζ����ߣ���ͼI��֪����ǡ���к�ʱ��������Һ��pH=7 | |
| B�� | ͼII��ʾһ�������½��еķ�Ӧ2SO2+O2?2SO3��H��0���ɷֵ����ʵ����仯��t2ʱ�̸ı�����������Ǽ�ѹ���¶� | |
| C�� | ͼIII��ʾij������Һ�м���Ba��OH��2��Һ�����������������Ba��OH��2��Һ����Ĺ�ϵ���ڼ���20ml Ba��OH��2��Һʱ��������Al��OH��3��BaSO4�Ļ���� | |
| D�� | ͼIV��ʾ��һ���������Ũ��NH4Cl��AlCl3��MgCl2�����Һ������NaOH��Һ�������Ĺ����У����ɳ��������������NaOH��Һ�����֮��Ĺ�ϵ |
�ش��������⣺
��1�����иĽ����Ż���ˮ�ۺ����ù��յ�������������е��Ǣڢۢܣ�����ţ���
���û�������ȡ��ˮ
����߲��ֲ�Ʒ������
���Ż���ȡ��Ʒ��Ʒ��
�ܸĽ��ء��塢þ�ȵ���ȡ����
��2�����á���������������Ũ��ˮ����Br2�����ô������գ���֪�÷�Ӧ������CO2�������绯ΪBr-��BrO3-����Ӧ�����ӷ�Ӧ����ʽΪ3Br2+6CO32-+3H2O=5Br-+BrO3-+6HCO3-��
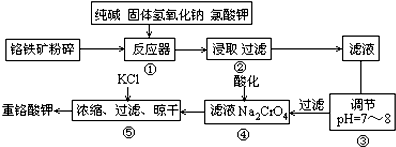
��3����ˮ��þ��һ�ι���������ͼ��

Ũ��ˮ����Ҫ�ɷ����£�
| ���� | Na+ | Mg2+ | Cl- | SO42- |
| Ũ��/��g•L-1�� | 63.7 | 28.8 | 144.6 | 46.4 |
��4����MgCl2•6H2O�Ʊ�MgCl2����ʱ�����Ȼ��������Χ�м��Ƚ��У���Ŀ��������MgCl2ˮ�⣮
 ���ڱ�����A��B��C����ԭ���������������Ԫ�أ�A�ǵؿ��к������Ľ���Ԫ�أ�B�ļ۲�����Ų�Ϊnsn-1npn+1��C�������е�һ�ֳ��ý�������ԭ�ӵ���Χ�����Ų�Ϊ3d104s1��
���ڱ�����A��B��C����ԭ���������������Ԫ�أ�A�ǵؿ��к������Ľ���Ԫ�أ�B�ļ۲�����Ų�Ϊnsn-1npn+1��C�������е�һ�ֳ��ý�������ԭ�ӵ���Χ�����Ų�Ϊ3d104s1��

 ��
�� ��
�� ��
�� ���ṹ��P�ĺ˴Ź���������5��壬P��ˮ������CH3CH2NH2�ͼף�1mol�������Ʒ�Ӧ����2mol H2����P�Ľṹ��ʽ��
���ṹ��P�ĺ˴Ź���������5��壬P��ˮ������CH3CH2NH2�ͼף�1mol�������Ʒ�Ӧ����2mol H2����P�Ľṹ��ʽ�� ��
��