��Ŀ����
ij�¶��£�C��H2O(g)���ܱ�����������з�Ӧ��
C(s)+H2O(g) CO(g)+H2(g)�� CO(g)+H2O(g)
CO(g)+H2(g)�� CO(g)+H2O(g) CO2(g)+H2(g)
CO2(g)+H2(g)
����Ӧ�ﵽƽ��ʱ��c(H2)="1." 9 mol��L��1,c(CO)="0." 1 mol��L��1��������������ȷ����
C(s)+H2O(g)
 CO(g)+H2(g)�� CO(g)+H2O(g)
CO(g)+H2(g)�� CO(g)+H2O(g) CO2(g)+H2(g)
CO2(g)+H2(g)����Ӧ�ﵽƽ��ʱ��c(H2)="1." 9 mol��L��1,c(CO)="0." 1 mol��L��1��������������ȷ����
| A��CO��ƽ��ʱת����Ϊ10% |
| B��ƽ��Ũ��c(CO2)=c(H2) |
| C��ƽ��ʱ����ƽ����Է�������ԼΪ23.3 |
| D��������������ʱ����С�����H2O(g)��ת������֮���� |
D
C(s)+H2O(g) CO(g)+H2(g)�� CO(g)+H2O(g)
CO(g)+H2(g)�� CO(g)+H2O(g) CO2(g)+H2(g)
CO2(g)+H2(g)
x x y y
��x��y��0.1mol/L��x+y��1.9mol/L�����x��1.0mol/L��y��0.9mol/L������CO��ƽ��ʱת����Ϊ90%��ƽ��Ũ��c(CO2)=0.9mol/L��B����ȷ������ˮ���������ʵ�����ȷ����C����ȷ������ѹǿ����һ����Ӧ���淴Ӧ�����ƶ�������H2O(g)��ת������֮���͡���ѡD��
 CO(g)+H2(g)�� CO(g)+H2O(g)
CO(g)+H2(g)�� CO(g)+H2O(g) CO2(g)+H2(g)
CO2(g)+H2(g)x x y y
��x��y��0.1mol/L��x+y��1.9mol/L�����x��1.0mol/L��y��0.9mol/L������CO��ƽ��ʱת����Ϊ90%��ƽ��Ũ��c(CO2)=0.9mol/L��B����ȷ������ˮ���������ʵ�����ȷ����C����ȷ������ѹǿ����һ����Ӧ���淴Ӧ�����ƶ�������H2O(g)��ת������֮���͡���ѡD��

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ
 2NH3(g)+ 92.4 kJ��ʵ������ʼ��ƽ��ʱ���й��������±���
2NH3(g)+ 92.4 kJ��ʵ������ʼ��ƽ��ʱ���й��������±��� cC(g) + dD(g) ��H=Q������ͼ�ش�
cC(g) + dD(g) ��H=Q������ͼ�ش�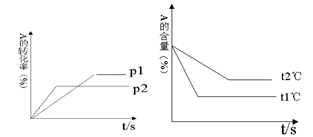
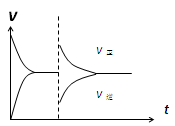
 2C(g)�ﵽ��ѧƽ��״̬�ı�־��
2C(g)�ﵽ��ѧƽ��״̬�ı�־�� Ag2O(s)��SO3(g),2SO3(g)
Ag2O(s)��SO3(g),2SO3(g) 2SO2(g)��O2(g)��10min��Ӧ�ﵽƽ�⣬��ʱc(SO3)Ϊ0.4mol��L��1��c(SO2)Ϊ0.1mol��L��1�������������в���ȷ����(����)
2SO2(g)��O2(g)��10min��Ӧ�ﵽƽ�⣬��ʱc(SO3)Ϊ0.4mol��L��1��c(SO2)Ϊ0.1mol��L��1�������������в���ȷ����(����)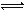 Z(g)����60s�ﵽƽ�⣬����0.3 mol Z��������ȷ����( )
Z(g)����60s�ﵽƽ�⣬����0.3 mol Z��������ȷ����( )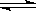 2SO3��g����ÿ�ʼʱ��SO2��O2����SO3��Ũ�ȷֱ�Ϊ0.2mol?L-1��0.1mol?L-1��0.2mol?L-1���ڷ�Ӧ�����в��ϲ�������и����ʵ�Ũ�ȣ����вⶨ���ݿ϶�����ȷ����
2SO3��g����ÿ�ʼʱ��SO2��O2����SO3��Ũ�ȷֱ�Ϊ0.2mol?L-1��0.1mol?L-1��0.2mol?L-1���ڷ�Ӧ�����в��ϲ�������и����ʵ�Ũ�ȣ����вⶨ���ݿ϶�����ȷ���� 3C(g),ֻ�з�Ӧ����ƽ��ʱ�ž��е������ǣ�������
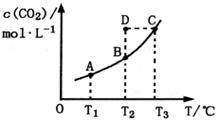
3C(g),ֻ�з�Ӧ����ƽ��ʱ�ž��е������ǣ������� CO2(g)+H2(g)��CO2��ƽ�����ʵ���Ũ��c(CO2)���¶�T�Ĺ�ϵ��ͼ��ʾ������˵��������ǣ� ��
CO2(g)+H2(g)��CO2��ƽ�����ʵ���Ũ��c(CO2)���¶�T�Ĺ�ϵ��ͼ��ʾ������˵��������ǣ� ��