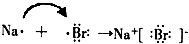��Ŀ����
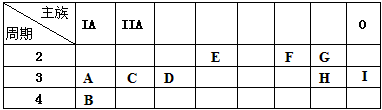
�±��г���A-I����Ԫ�������ڱ��е�λ�ã�
��1�������Ԫ���У���������ǿ��Ԫ����______����Ԫ�ط��ţ���ͬ������ѧ��������õ�Ԫ����______��
��2��A��C��D����Ԫ�ص��������Ӧ��ˮ������м�����ǿ����______���ѧʽ��
��3��A��B��C��D����ԭ��ԭ�Ӱ뾶�ɴ�С��˳������Ϊ______����Ԫ�ط�������A��G���γɵ��Ӳ�ṹ��ͬ�����ӣ����������ӵİ뾶�ɴ�С����Ϊ______���ѧʽ����
��4��FԪ�ص��⻯����______��______������һ������B�ĵ��ʷ�����Ӧ����д���÷�Ӧ�����ӷ���ʽ______��
��5�������Ԫ��֮������γɶ��ּ����ӻ�������ۻ������д������һ�ֵĻ�ѧʽ______�����õ���ʽ��ʾ���γɹ��̣�______��
| ���� ���� |
IA | IIA | 0 | |||||
| 2 | E | F | G | |||||
| 3 | A | C | D | H | I | |||
| 4 | B |
��2��A��C��D����Ԫ�ص��������Ӧ��ˮ������м�����ǿ����______���ѧʽ��
��3��A��B��C��D����ԭ��ԭ�Ӱ뾶�ɴ�С��˳������Ϊ______����Ԫ�ط�������A��G���γɵ��Ӳ�ṹ��ͬ�����ӣ����������ӵİ뾶�ɴ�С����Ϊ______���ѧʽ����
��4��FԪ�ص��⻯����______��______������һ������B�ĵ��ʷ�����Ӧ����д���÷�Ӧ�����ӷ���ʽ______��
��5�������Ԫ��֮������γɶ��ּ����ӻ�������ۻ������д������һ�ֵĻ�ѧʽ______�����õ���ʽ��ʾ���γɹ��̣�______��
����Ԫ�������ڱ��е�λ�ã�������֪AΪNa��BΪK��CΪMg��DΪAl��EΪC��FΪO��GΪF��HΪCl��IΪAr��
��1��Ԫ�����ڱ��У����ϵ��£�Ԫ�صĽ���������ǿ�����ҵ���Ԫ�صĽ���������ǿ�����Խ�������ǿ�Ľ��������½ǣ���ΪK��ϡ������Ԫ�ص�ԭ�ӻ�ѧ��������ã�
�ʴ�Ϊ��K��Ar��
��2��Na��Mg��Al��ͬ����Ԫ�ص�ԭ�ӣ������ң�Ԫ�ص�����������Ӧ��ˮ����ļ����������ʼ�����ǿ�����������ƣ���Ϊ��NaOH��
��3��K���Ӳ���࣬�뾶���Na��Mg��Al��ͬ����Ԫ�ص�ԭ�ӣ�������ԭ�Ӱ뾶��С����Na��Mg��Al�������Ӻͷ����Ӿ�����ͬ�ĵ����Ų���������Խ��뾶ԽС����F-��Na+���ʴ�Ϊ��K��Na��Mg��Al��F-��Na+��
��4����Ԫ�ص���������ˮ��˫��ˮ��K��ˮ��Ӧ�����������غ��������ʴ�Ϊ��H2O��H2O2��2K+2H2O=2K++2OH-+H2����
��5�����ý����ͻ��÷ǽ���֮���γɵĻ�������Ϊ���ӻ�����õ���ʽ��ʾNaCl�γɹ���Ϊ

��
�ǽ���֮���γɵĻ�������Ϊ���ۻ������H2O��HCl�ȣ�H2O�и�ԭ�Ӵﵽ�ȶ��ṹ���õ���ʽ��ʾ�γɹ���Ϊ
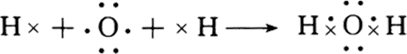
��
�ʴ�Ϊ��NaCl��H2O����

��
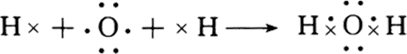
����
��1��Ԫ�����ڱ��У����ϵ��£�Ԫ�صĽ���������ǿ�����ҵ���Ԫ�صĽ���������ǿ�����Խ�������ǿ�Ľ��������½ǣ���ΪK��ϡ������Ԫ�ص�ԭ�ӻ�ѧ��������ã�
�ʴ�Ϊ��K��Ar��
��2��Na��Mg��Al��ͬ����Ԫ�ص�ԭ�ӣ������ң�Ԫ�ص�����������Ӧ��ˮ����ļ����������ʼ�����ǿ�����������ƣ���Ϊ��NaOH��
��3��K���Ӳ���࣬�뾶���Na��Mg��Al��ͬ����Ԫ�ص�ԭ�ӣ�������ԭ�Ӱ뾶��С����Na��Mg��Al�������Ӻͷ����Ӿ�����ͬ�ĵ����Ų���������Խ��뾶ԽС����F-��Na+���ʴ�Ϊ��K��Na��Mg��Al��F-��Na+��
��4����Ԫ�ص���������ˮ��˫��ˮ��K��ˮ��Ӧ�����������غ��������ʴ�Ϊ��H2O��H2O2��2K+2H2O=2K++2OH-+H2����
��5�����ý����ͻ��÷ǽ���֮���γɵĻ�������Ϊ���ӻ�����õ���ʽ��ʾNaCl�γɹ���Ϊ

��
�ǽ���֮���γɵĻ�������Ϊ���ۻ������H2O��HCl�ȣ�H2O�и�ԭ�Ӵﵽ�ȶ��ṹ���õ���ʽ��ʾ�γɹ���Ϊ
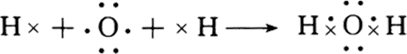
��
�ʴ�Ϊ��NaCl��H2O����

��
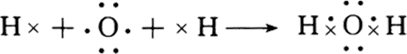
����

��ϰ��ϵ�д�
�����Ŀ
�±��г���A-I����Ԫ�������ڱ��е�λ�ã�
��1�������Ԫ���У���������ǿ��Ԫ����______����Ԫ�ط��ţ���ͬ������ѧ��������õ�Ԫ����______��
��2��A��C��D����Ԫ�ص��������Ӧ��ˮ������м�����ǿ����______���ѧʽ��
��3��A��B��C��D����ԭ��ԭ�Ӱ뾶�ɴ�С��˳������Ϊ______����Ԫ�ط�������A��G���γɵ��Ӳ�ṹ��ͬ�����ӣ����������ӵİ뾶�ɴ�С����Ϊ______���ѧʽ����
��4��FԪ�ص��⻯����______��______������һ������B�ĵ��ʷ�����Ӧ����д���÷�Ӧ�����ӷ���ʽ______��
��5�������Ԫ��֮������γɶ��ּ����ӻ�������ۻ������д������һ�ֵĻ�ѧʽ______�����õ���ʽ��ʾ���γɹ��̣�______��
| ���� ���� | IA | IIA | ||||||
| 2 | E | F | G | |||||
| 3 | A | C | D | H | I | |||
| 4 | B |
��2��A��C��D����Ԫ�ص��������Ӧ��ˮ������м�����ǿ����______���ѧʽ��
��3��A��B��C��D����ԭ��ԭ�Ӱ뾶�ɴ�С��˳������Ϊ______����Ԫ�ط�������A��G���γɵ��Ӳ�ṹ��ͬ�����ӣ����������ӵİ뾶�ɴ�С����Ϊ______���ѧʽ����
��4��FԪ�ص��⻯����______��______������һ������B�ĵ��ʷ�����Ӧ����д���÷�Ӧ�����ӷ���ʽ______��
��5�������Ԫ��֮������γɶ��ּ����ӻ�������ۻ������д������һ�ֵĻ�ѧʽ______�����õ���ʽ��ʾ���γɹ��̣�______��
 ��
��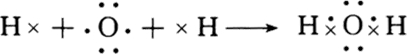 ��
��