��Ŀ����
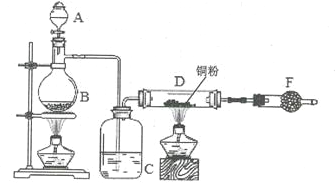
����Ŀ�����ǻ����ʰ�����������Ҫ���л��м��壬ij�������ԶԼ�����Ϊԭ����ƶ��ǻ����ʰ��������ĺϳ�·����ͼ��ʾ(���ַ�Ӧ��������ȥ)��

��֪��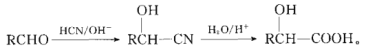
�ش��������⣺
��1��B�к��������ŵ�������_____________��
��2���ݡ���ķ�Ӧ���ͷֱ���______________��_______________��
��3��E�Ľṹ��ʽ��__________________��
��4����Ӧ�۵Ļ�ѧ����ʽΪ___________________��
��5����Ӧ��Ļ�ѧ����ʽΪ_____________(����ע����Ӧ����)��
��6��C��ͬ���칹���ж��֣����к��б��������������ͬ���칹����____��(�����������칹)�����к˴Ź���������ʾֻ��4����Ľṹ��ʽΪ______________(��дһ��)��
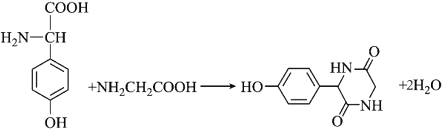
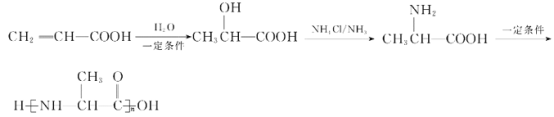
��7�����������ϳ�·�ߺ���Ϣ��д����CH2=CHCOOHΪԭ��(���Լ���ѡ)���Ʊ� �ĺϳ�·�ߣ�________��
�ĺϳ�·�ߣ�________��
���𰸡��ǻ����ѻ� �ӳɷ�Ӧ ȡ����Ӧ 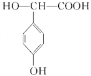
 6
6 ![]()
��������
�۲�D�����ķ�Ӧ����֪��Ϣ��֪�����Ʋ�����D�Ľṹ��ʽΪ��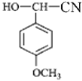 ������C��Ϊ��
������C��Ϊ��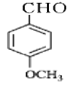 ������BΪ
������BΪ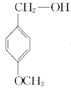 ��
��
��1��B�Ľṹ��ʽΪ��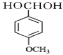 �����������ŵ��������ǻ����ѻ���
�����������ŵ��������ǻ����ѻ���
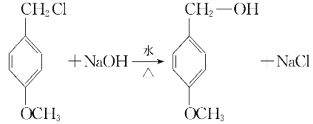
��2���ݷ�Ӧ��C����ȩ�������跢���ӳɷ�Ӧ����D����ķ�ӦΪE�еĴ��ǻ��백������ȡ����Ӧ����Ϊ���ӳɷ�Ӧ��ȡ����Ӧ��
��3���߷�Ӧ����EΪ�ѻ���Ϊ���ǻ��ķ�Ӧ����E�Ľṹ��ʽΪ�� ��
��
��4����Ӧ��Ϊ±������ˮ�ⷴӦ����Ӧ����ʽΪ�� ��
��
��5��F�к����Ȼ��Ͱ�������![]() ��Ӧ�����ļ�����Ӧ����ʽΪ��
��Ӧ�����ļ�����Ӧ����ʽΪ��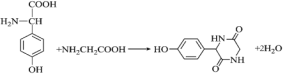 ��
��
��6��C�ķ���ʽΪC8H8O2��2��ȡ����ʱ��-CH3��-OOCH�����ڼ��3�֣�һ��ȡ����-OOC CH3��-CH2OOCH��-COOCH3������6�֣��˴Ź���������ʾֻ��4����Ϊ�Գƽṹ��ֻ��1��ȡ�������Һ�1����ԭ�ӣ���Ϊ��6��![]() ��
��
��7�����������ϳ�·�߷�Ӧ����Ϣ���ǻ�����ת��Ϊ��������CH2=CHCOOH��ˮ�����ӳɷ�Ӧ���ɼ����ǻ����ھ�����Ӧ�༴����ȡ�����ᣬ�ٷ������۷�Ӧ���ɣ���Ϊ�� ��
��