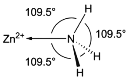
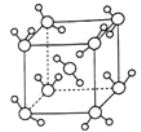
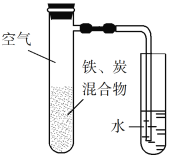
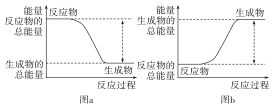
��Ŀ����
����Ŀ�������к��зḻ�ĵ⣬Ϊ�˴Ӻ�������ȡ�⣬ij�о���ѧϰС����Ʋ�����������ʵ�飺
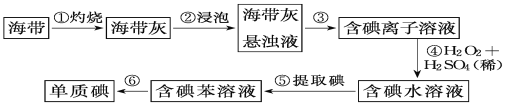
����д���пհף�
��1������������պ���ʱ������Ҫ���ż��⣬����Ҫ�õ���ʵ��������___��___��___��___��
��2������۵�ʵ�����������__�������Ŀ���ǴӺ��ⱽ��Һ�з�������ʵ�ͻ��ձ����ò����ʵ�����������__��
��3������ܷ�Ӧ�����ӷ���ʽ��___��
��4��������У�ijͬѧѡ���ñ�����ȡ���������___��
��5�������һ�ּ�����ȡ����ˮ��Һ���Ƿ��е��ʵ�ļ�����___��
���𰸡����� ����ǯ ������ �ƾ��� ���� ���� 2I-��H2O2��2H+=I2��2H2O ����ˮ�������ܣ����ڱ��е��ܽ�ȱ���ˮ�д� ȡ������ȡ����ˮ��Һ���Թ��У����뼸�ε�����Һ���۲��Ƿ������ɫ�����������˵�����е��ʵ�
��������
ʵ���ҴӺ�������ȡ�⣺�ٺ������ճɻң��ڽ����ܽ�õ�����������Һ����ͨ�����ˣ��õ����ܵIJ�������ҺΪ�������ӵ���Һ���ܼ������������H2O2���������������ɵⵥ�ʣ��������л��ܼ�����ȡ���ⵥ�ʣ�����ͨ��������ȡ���ⵥ�ʡ�
��1�����պ�����Ϊ����ļ��ȣ�Ӧ�������н��У�����Ҫ���ż��⣬����Ҫ����������ǯ�������ǡ��ƾ��ƣ�
��2��������Ƿ�������Һ�壬��ʵ�����Ϊ���ˣ������Ŀ���ǴӺ��ⱽ��Һ�з�������ʵ�ͻ��ձ��������û��ܵ�����Һ��ķе㲻ͬ�����룬��ʵ�����Ϊ����
��3��������ǵ����������������±�H2O2�������ɵ��ʵ⣬��Ӧ�����ӷ���ʽΪ2I- + H2O2 + 2H+ = I2 + 2H2O��
��4������ݵIJ���Ϊ��ȡ��������ȡ��ѡ���һ��ԭ����������ȡ������ܽ��Ҫ������ȡ����ԭ�ܼ��������ܣ�����I2�����ڱ����ұ���ˮ�������ܣ��ʿ��ñ�����ȡ�⣬��˴�Ϊ������ˮ�������ܣ����ڱ��е��ܽ�ȱ���ˮ�д�
��5���ⵥ������۷�Ӧ����ɫ����˿��������ʵ�飺ȡ������ȡ����ˮ��Һ���Թ��У����뼸�ε�����Һ���۲��Ƿ������ɫ�����������˵�����е��ʵ⡣

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�