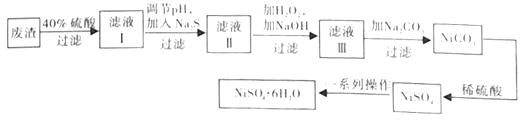
��Ŀ����
����Ŀ��Ԫ�ظ�(Cr)����Ȼ����Ҫ��+3�ۺ�+6�۴��ڡ���ش��������⣺
(1)���ø�����(FeOCr2O3)ұ����ȡ�������Ĺ���������ͼ��ʾ��
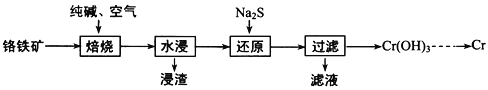
��Ϊ�ӿ챺�����ʺ����ԭ�ϵ������ʣ��ɲ�ȡ�Ĵ�ʩ֮һ��__________________ ��
����ˮ����Ҫ��ý���Һ�IJ�����_________������Һ����Ҫ�ɷ�ΪNa2CrO4��������Һ���м����ữ���Ȼ�����Һ�а�ɫ�������ɣ��� ����ԭ�������з�����Ӧ�����ӷ���ʽΪ___________________________________________________��
(2)��֪ Cr3+��ȫ����ʱ��ҺpHΪ5��(Cr3+Ũ�Ƚ���10-5molL-1����Ϊ��ȫ����)��Cr(OH)3���ܶȻ����� Ksp��_______________��
(3)��ʯī�缫��������(Na2CrO4)��Һ�������ظ�����(Na2Cr2O7)��ʵ��װ����ͼ��ʾ(��֪��2CrO42-+2H+![]() Cr2O72-+ H2O)��
Cr2O72-+ H2O)��
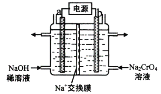
�ٵ缫b���ӵ�Դ��______��(����������������) �� b �������ĵ缫��Ӧ��______________��
�ڵ��һ��ʱ������������Һ��Na+���ʵ�����a mol��Ϊb mol���������������ظ����Ƶ����ʵ�����_______________mol ��
���𰸡���������� ���� 8CrO42��+3S2-+20H2O=8Cr(OH)3��+3SO42-+16OH- 1��10-32 �� 2H2O-4e-=O2��+4H+ (a-b)/2
��������
(1)�������̣�������(FeOCr2O3)���봿�ͨ��������գ��õ�Na2CrO4�����ˣ���Һ����Na2CrO4������Na2S��ԭ�õ�Cr(OH)3����ӦΪ��8CrO42-+3S2-+20H2O=8Cr(OH)3��+3SO42-+16OH-������Cr(OH)3�ɵõ�Cr2O3�����û�ԭ����ԭ�õ�Cr���ݴ˷������
(2)��֪Cr3+��ȫ����ʱ��ҺpHΪ5�����ʱc(OH-)=1��10-9mol/L��c(Cr3+)=1��10-5mol/L��Cr(OH)3���ܶȻ�����Ksp=c(Cr3+)c3(OH-)���ݴ˼��㣻
(3)��b���õ�Na2Cr2O7����b�з�Ӧ2CrO42-+2H+![]() Cr2O72-+H2O����b�ĵ缫��ӦΪˮ�ŵ����������������ӣ��ݴ˷�����𣻢ڸ��ݵ���غ�������㡣
Cr2O72-+H2O����b�ĵ缫��ӦΪˮ�ŵ����������������ӣ��ݴ˷�����𣻢ڸ��ݵ���غ�������㡣
(1)��Ӱ�컯ѧ��Ӧ���ʵ����أ����ʵı������С�������Խ��Ӧ����Խ�죬Ϊ�ӿ챺�����ʺ����ԭ�ϵ������ʣ���ȡ�Ĵ�ʩ�����н����������ȣ��ʴ�Ϊ����������飻
����ˮ����Ҫ��ý���Һ����Ҫ���˳���������ȡ�IJ����ǹ��ˣ�����Һ����Ҫ�ɷ�ΪNa2CrO4��������Һ���м����ữ���Ȼ�����Һ�а�ɫ�������ɣ�˵�������Na2S��������SO42-��CrԪ����+6�۽�Ϊ+3�ۣ�SԪ����-2������+6�ۣ�����ԭ���غ�͵�ʧ�����غ㣬��Ӧ�ķ���ʽΪ��8CrO42��+3S2-+20H2O=8Cr(OH)3��+3SO42-+16OH-���ʴ�Ϊ�����ˣ�8CrO42��+3S2-+20H2O=8Cr(OH)3��+3SO42-+16OH-��
(2)pHΪ5��c(OH-)=10-9��c(Cr3+)=1��10-5mol/L����Ksp��c(Cr3+)c3(OH-)=10-5��(10-9)3=1��10-32���ʴ�Ϊ��1��10-32��
(3)�ٸ���ͼʾ����b�����ڵ缫�ҵõ�Na2Cr2O7������2CrO42-+2H+![]() Cr2O72��+ H2O����������b��c(H+)������b���缫��ӦʽΪ2H2O-4e-=O2��+4H+�����a��������b���������缫b���ӵ�Դ���������ʴ�Ϊ������2H2O-4e-=O2��+4H+��
Cr2O72��+ H2O����������b��c(H+)������b���缫��ӦʽΪ2H2O-4e-=O2��+4H+�����a��������b���������缫b���ӵ�Դ���������ʴ�Ϊ������2H2O-4e-=O2��+4H+��
�ڵ��һ��ʱ������������Һ��Na+���ʵ�����a mol��Ϊb mol������Һ���ƶ��ĵ��Ϊ(a-b)mol���������·��ת�Ƶĵ���Ϊ(a-b)mol�������ĵ缫��ӦΪ��2H2O-4e-=O2+4H+�����������ɵ�������Ϊ(a-b)mol������2CrO42-+2H+Cr2O72-+H2O�������������ظ����Ƶ����ʵ�����![]() mol���ʴ�Ϊ��
mol���ʴ�Ϊ��![]() ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�