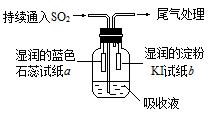
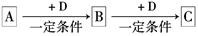��Ŀ����
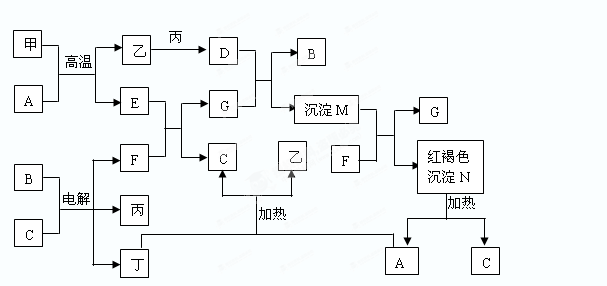
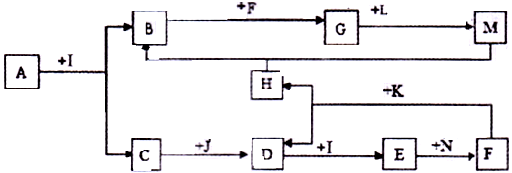
��ͼ����ѧ��ѧijЩ����֮����һ�������µ��ת����ϵ����֪A��һ�ֳ�����Һ̬������,C��D��G��H��K�ǵ��ʣ�����Ϊ�����G��K����ͨ���е�������ҪԪ�أ�����K�����٣�E��F��Ӧ��Һ��������෴��F����ɫ��ӦΪ��ɫ���밴Ҫ������
��1��д��������ĵ���ʽ��_________________________��
��2����д��A��J��Ӧ�����ӷ���ʽ��_______________________________________��
�ڽ�������Hͨ��F����Һ�У���Ӧ�������ӷ���ʽΪ��_________________________��
��3��B������ϡ���ᷴӦ�����μӷ�Ӧ������Ϊ4mol��ת�Ƶ��ӵ����ʵ���Ϊ_________mol������2λ��Ч���֣���
��4����֪��200�棬101Kpa�£�0��12g����K��A��ȫ��Ӧ����C��I��������1316J��������д���˷�Ӧ���Ȼ�ѧ����ʽ��_________________________________________��
��5������PtΪ�缫����I��D�Լ�F����Һ���ԭ��أ��������ĵ缫��ӦΪ��___________��
�����ô˵�ص��300mL5mol��L���Ȼ�����Һһ��ʱ�䣬�������ռ�����״����3��36L���壬��ʱ��Һ��pHΪ_________��������ǰ����Һ������仯����
�������õ���������Һ��ͨ�������̼����4��48L����״��������ʱ��Һ����������Ũ�ȵĹ�ϵ�ɴ�С��˳��Ϊ��_______________________________________��
(1)
(2) ��2H2O+2Na2O2=" 4" Na��+4OH��+ O2�� ��2F2+4 OH��= 4F��+ O2+2H2O
(3)0.43
(4)C(s)+H2O(g)=CO(g)+H2(g) ?H=+131.6kJ/mol������2000C,101Kpa��ע����һ�֣�״̬��������֣�+©�������֣���2�֣�
(5)��O2+4e��+2H2O = 4OH�� ��2�֣�
��14 ��2�֣�
��c(Na��)��c(Cl��)��c(HCO3��) ��c(CO32��)��c(OH��)��c(H��) ��2�֣� (©д���Ӳ�����)
�����������������������Ƴ�A+H�ķ�ӦΪ2F2+2H2O=4HF+O2��A+G�ķ�ӦΪ3Fe+4 H2O=Fe3O4 +4H2�� A+K�ķ�ӦΪC+ H2O="CO" + H2.A+J�ķ�ӦΪ2Na2O2 +2H2O="4NaOH+" O2�����������ش����1����2�����ʡ���3��Fe3O4��HNO3��Ӧʱת�Ƶĵ�����Ϊ4*��1/28��*3��0.43mol.(4) C(s)+H2O(g)=CO(g)+H2(g) ?H="+131.6kJ/mol(5)" ��O2��������O2+4e��+2H2O = 4OH����2NaCl + 2H2O ="2NaOH" + H2 + Cl2 .ͨ������������������Ϊ3��36L�������NaOH�����ʵ���0.3mol���������NaOH��Ũ��Ϊ1mol/L��pH="14." ��ͨ��4��48L������̼����Һ����1.2molNaCl��Na2CO3Ϊ0.1mol ,NaHCO3Ϊ0.1mol������CO32-ˮ����������HCO3-�������Ƴ������ӵ�Ũ�ȴ�С��ϵ��
���㣺�������ʵ������ƶϺͷ�Ӧԭ���Ŀ��졣

���б仯�У������ڻ�ѧ�仯����
| A��SO2ʹƷ����ɫ | B����ˮʹ��ɫ������ɫ |
| C������̿ʹ��īˮ��ɫ | D��Ư��ʹijȾ����ɫ |