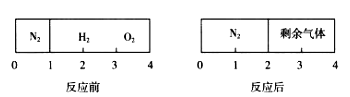
��Ŀ����
����Ŀ��25��ʱ��������ĵ���ƽ�ⳣ�����±���ʾ��
��ѧʽ | CH3COOH | HClO | H3PO3 |
���� | ���� | ������ | ������ |
����ƽ�ⳣ�� | 1.8��10-5 | 3.0��10-8 | K1=8.3��10-3 K2 =5.6��10-6 |
�ش�����������
��1��Ũ�Ⱦ�Ϊ0.1 molL-1��CH3COOH ��HClO ��H3PO3��Һ����c(H+)��С����_____________��
��2��������(H3PO3)Ϊ��Ԫ�ᣬ���н�ǿ�Ļ�ԭ�ԡ�H3PO3�ĵڶ������뷽��ʽΪ________________��Na2HPO3��_____________(������ʽ��������ʽ��������������)��
��3����������0.1 molL-1��CH3COOH��Һ��ˮϡ�͵Ĺ����У����б���ʽ����ֵ������______������ĸ����
A.c(H+) B. 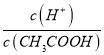 C.c(CH3COO-) D.c(CH3COOH)
C.c(CH3COO-) D.c(CH3COOH)
��4�������Ϊ10mL��c(H+)��Ϊ10-2 mol L-1�Ĵ�����Һ��һԪ��HX�ֱ��ˮϡ����1000 mL��ϡ������c(H+)�ı仯��ͼ��ʾ����HX�ĵ���ƽ�ⳣ��_______________(������������ С��������������)����ĵ���ƽ�ⳣ����������____________________________��
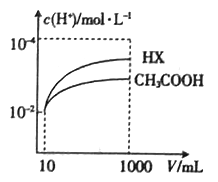
���𰸡� HC1O��Һ H2PO3-![]() HPO32-+H+ ���� B ���� c(H+)��ͬ�Ĵ����HX��Һϡ����ͬ�ı�����HX��Һ��c (H+)�仯����
HPO32-+H+ ���� B ���� c(H+)��ͬ�Ĵ����HX��Һϡ����ͬ�ı�����HX��Һ��c (H+)�仯����
����������1���ݵ���ƽ�ⳣ����֪����������ǿ������˳��Ϊ�����ᡢ���ᡢ�����ᣬ����Ũ�Ⱦ�Ϊ0.1 molL-1��CH3COOH��HClO��H3PO3��Һ�У�c(H+)��С����HClO����2��������Ϊ��Ԫ��ǿ�ᣬ�ֲ������ҵ�����棬����H3PO3�ĵڶ������뷽��ʽΪH2PO3-![]() HPO32-+H+��Na2HPO3�����ٵ���������ӣ��������Σ���3��A��CH3COOH��Һ��ˮϡ���̣��ٽ����룬c(H+)��С��A����B��
HPO32-+H+��Na2HPO3�����ٵ���������ӣ��������Σ���3��A��CH3COOH��Һ��ˮϡ���̣��ٽ����룬c(H+)��С��A����B�� 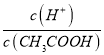 =n��H+��/n��CH3COOH������ϡ�����б�ֵ���B��ȷ��C��ϡ���̣��ٽ����룬c(CH3COO-)��С��C����D��ϡ���̣��ٽ����룬c(CH3COOH)��С��D����ѡB����4����ͼ��֪��ϡ����ͬ�ı�����HX��pH�仯�̶ȴ�������HXǿ������ƽ�ⳣ����
=n��H+��/n��CH3COOH������ϡ�����б�ֵ���B��ȷ��C��ϡ���̣��ٽ����룬c(CH3COO-)��С��C����D��ϡ���̣��ٽ����룬c(CH3COOH)��С��D����ѡB����4����ͼ��֪��ϡ����ͬ�ı�����HX��pH�仯�̶ȴ�������HXǿ������ƽ�ⳣ����

����Ŀ����ѧʵ����ѧϰ���⻯ѧ֪ʶ�Ļ���,�����������������ͷḻ���ں��ڻ�ѧѧϰ�з����Ŷ��صĹ��ܺ����á���ش��������⣺
I .ʵ�������ù���NaOH����100 mL 1mol��L-1��NaOH��Һ��
��1������������Һ�����в����õ���������____________________(����Żش�)��
A.�ձ� B.���Թ� C.��ͷ�ι� D.100 mL����ƿ
��2������������������һ��Ҫ�IJ���������______________��
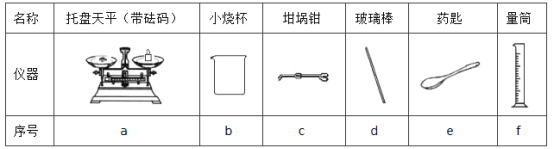
��3����������ƽ��ȡNaOH���������Ϊ_________g�����±���ѡ�����NaOH��������Ҫ������ __________________�����������
��4���������ʹ�������Һ��Ũ����α仯��������ƫ������ ƫ����������������
A.δϴ���ܽ��������Ƶ��ձ�_________��
B.����ƿʹ��ǰ������ˮϴ�����ڱڸ���ˮ���δ���ﴦ��___________��
C.����ʱ��ˮ�����˿̶��ߣ��������Һ������___________��
D.����ʱ��������ƿ����___________��
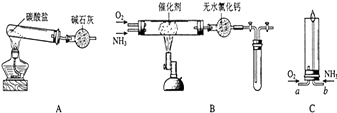
II����֪��������Ҷ�������ˮ������������������ڻ����98%��Ũ���ᣬ���Ҳ��ܡ�����һ�ݼ��ҵĻ������Ʒ��ͨ��ʵ����з��룬�ɵõ��������ʵ����ʹ�õĹ����������ڹ���ǿ����Һ���������������������������ѧ��Ӧ����
����д���пհף���������������õ������ʵ����ơ�
��� | ʵ�鲽�� | ����ʵ��������������������װʵ��װ�ã� |
�� | �ܽ� | �����������ձ��У�����98% H2SO4____�� |
�� | _____________ | |
�� | ϡ���������� | ____________ |
�� | ���� | |
�� | _________ | �����Ĺ�������ע����������ˮ��ʹˮ������������ˮ�˳����ٴμ�ˮϴ�ӣ���ϴ���Ρ� |
�� | ��������Ƿ�ϴ�� | ____________________�� |