��Ŀ����
����Ŀ����ͼװ����ʾ��C��D��E��F��X��Y��Ϊ���Ե缫���ס�������Һ�����Ϊ500mL����ҺŨ�Ⱦ���ͬ(����ͨ��ǰ����Һ�������)��A��BΪ���ֱ����Դ����������ֱ����Դ��ͨ��F�������ʺ�ɫ����ش�

��1��B���ǵ�Դ��_____����һ��ʱ�������Һ��ɫ��____������X����������ɫ��dz��Y����������ɫ��������Fe(OH)3��������____�ɡ�
��2�����һ��ʱ���ס���װ���е�C��D��E��F�缫���ֱ�ֻ��һ�ֵ�������ʱ����Ӧ���ʵ����ʵ���֮��Ϊ______________�����з�����Ӧ�Ļ�ѧ����ʽΪ__________________________________����Ҫʹ����ļ�װ���е���Һ�ָ���ԭŨ���������һ������_________(�ѧʽ)��
��3�����ñ�װ�ø�ͭ����������HӦ����______(�����Ʋ�����������Ƽ���)�����Ƽ�������5.4g��ʱ�� �ס�������Һ��pH�ֱ�Ϊ_____��_____
���𰸡� �� dz �� 1:2:2:2 2NaCl��2H2O![]() 2NaOH��H2����Cl2�� CuO �Ƽ� 1 13
2NaOH��H2����Cl2�� CuO �Ƽ� 1 13
��������������������⿼���ش���װ�ã��漰װ�õķ���������ĵ�Ӿ���绯ѧ�ļ��㣬��ơ���ͨ��Դ��F�������ʺ�ɫ��FΪ������EΪ��������AΪ��Դ��������BΪ��Դ�ĸ�����
��1��B���ǵ�Դ�ĸ�����C��DΪ���Ե缫������ΪCuSO4��Һ�����е�ⷴӦ����ʽΪ2CuSO4+2H2O![]() 2Cu+2H2SO4+O2��������Cu2+Ũ�ȼ�С��������Һ����ɫ��dz������ͼʾXΪ������YΪ������Y����������ɫ�������Fe��OH��3������������ɡ�
2Cu+2H2SO4+O2��������Cu2+Ũ�ȼ�С��������Һ����ɫ��dz������ͼʾXΪ������YΪ������Y����������ɫ�������Fe��OH��3������������ɡ�
��2��CΪ������C���ĵ缫��ӦʽΪ4OH--4e-=O2��+2H2O��DΪ������D���ĵ缫��ӦʽΪCu2++2e-=Cu��EΪ������E���ĵ缫��ӦʽΪ2Cl--2e-=Cl2����FΪ������F���ĵ缫��ӦʽΪ2H++2e-=H2�������ݵ�·��ͨ���������ʵ�����ȣ�C��D��E��F���ɵĵ������ʵ���֮��Ϊ1:2:2:2�����з�����Ӧ�Ļ�ѧ����ʽΪ2NaCl+2H2O![]() 2NaOH+H2��+Cl2������װ���з����ķ�ӦΪ2CuSO4+2H2O
2NaOH+H2��+Cl2������װ���з����ķ�ӦΪ2CuSO4+2H2O![]() 2Cu+2H2SO4+O2��������ԭ���غ㣬Ҫʹ��װ���е���Һ�ָ���ԭŨ�������һ������CuO��
2Cu+2H2SO4+O2��������ԭ���غ㣬Ҫʹ��װ���е���Һ�ָ���ԭŨ�������һ������CuO��
��3����װ����GΪ������HΪ���������ݵ��ԭ������ͭ��������HΪ�Ƽ����Ƽ��ϵĵ缫��ӦʽΪAg++e-=Ag��n��Ag��=![]() =0.05mol����·��ͨ���ĵ������ʵ���Ϊ0.05mol�����з�ӦΪ2Cu2++2H2O
=0.05mol����·��ͨ���ĵ������ʵ���Ϊ0.05mol�����з�ӦΪ2Cu2++2H2O![]() 2Cu+4H++O2��~4e-����������n��H+��=n��e-��=0.05mol��c��H+��=
2Cu+4H++O2��~4e-����������n��H+��=n��e-��=0.05mol��c��H+��=![]() =0.1mol/L��������Һ��pH=1�����з�ӦΪ2Cl-+2H2O
=0.1mol/L��������Һ��pH=1�����з�ӦΪ2Cl-+2H2O![]() 2OH-+H2��+Cl2��~2e-����������n��OH-��=n��e-��=0.05mol��c��OH-��=
2OH-+H2��+Cl2��~2e-����������n��OH-��=n��e-��=0.05mol��c��OH-��=![]() =0.1mol/L��c��H+��=1
=0.1mol/L��c��H+��=1![]() 10-13mol/L��������Һ��pH=13��
10-13mol/L��������Һ��pH=13��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��ij��ȤС��ͬѧ��ʵ�����ü���1-������ŨH2SO4���廯�ƻ����ķ������Ʊ�1-�嶡�飬�����鷴Ӧ�IJ��ָ�����������ͼ��ʾװ�ã����мг�������������������ȴˮ��û�л�����
��֪��NaBr+H2SO4=NaHSO4+HBr
��CH3(CH2)2CH2OH+HBr![]() CH3(CH2)2CH2Br+H2O
CH3(CH2)2CH2Br+H2O
����Ӧ�������
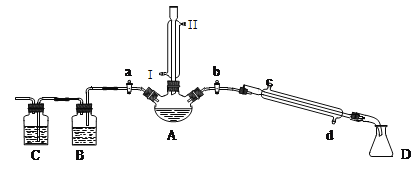
�����ʵ�鲽�裬�ش��������⣺
��1���ر�a��b����ͨ��ֱ�����ܵ�����ˮ����A����30���ӣ��Ʊ�1-�嶡�顣��ֱ�����ܽ�ͨ����ˮ����ˮ����_____(�I����)��������������ҪĿ����________��
��2�������ϣ�������Ӧ�ĸ���������У�����(CH3CH2CH2CH2-O-CH2CH2CH2CH3)��1-��ϩ���廯�⡢�������ơ�ˮ�ȡ�Ϩ��ƾ��ƣ�����ֱ�������Ϸ��������Ӳ���a���������ȼ�����Ӧֱ����ȴ��ͨ��B��Cװ�ü��鲿�ָ�����B��C��Ӧʢ�ŵ��Լ��ֱ���ʯ�����ˮ��Bװ�ó���ʯ�ﻹ����______(���Լ�����)д��Cװ������Ҫ�Ļ�ѧ����ʽ��_________________________________________________��
��ͬѧ��ͨ����������Ǽ������ò������Ƿ�������CH2CH2CH2CH3���� ��ȷ���������д��ڶ��ѡ�����Ը�ͬѧ�Ĺ۵��������________________________________��
��3��Ϊ�˽�һ�������ᴿ1-�嶡�飬����ȤС��ͬѧ�������л�������������ʾ��
���� | �۵�/�� | �е�/�� |
1-���� | -89.5 | 117.3 |
1-�嶡�� | -112.4 | 101.6 |
���� | -95.3 | 142.4 |
1-��ϩ | -185.3 | -6.5 |
���㲹������ʵ�鲽�裬ֱ�������1-�嶡�顣
�ٴ���ƿ��ȴ��ȥ��ֱ�������ܣ�
�ڲ��ϴ���Ƥ�����¶ȼƣ�
�۹ر�_______����_______��
�ܽ�ͨ�����ܵ�����ˮ��ʹ��ˮ��______�����룻
��Ѹ�������¶���_______�棬�ռ�������֡�
��4����ʵ������ȡ1-������NaBr�ֱ�Ϊ7.4 g��13.0 g�������Ĵֲ��ᆳϴ�ӡ�������ٴ�����õ�10.96 g 1-�嶡�飬��1-�嶡��IJ�����_____��������2λ��Ч���֣�
