��Ŀ����
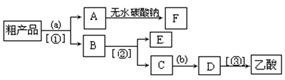
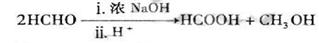
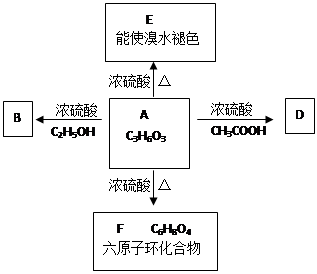
��֪����A��ʯ���ѽ�������Ҫ�ɷݣ�A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ����2CH3CHO+O2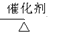 2CH3COOH������AΪ��Ҫԭ�Ϻϳ�������������ϳ�·������ͼ��ʾ��
2CH3COOH������AΪ��Ҫԭ�Ϻϳ�������������ϳ�·������ͼ��ʾ��

�ش��������⣺
��1��д��A�ĵ���ʽ ��
��2��B��D�����еĹ��������Ʒֱ��� �� ��
��3��д�����з�Ӧ�ķ�Ӧ���ͣ��� ���� ���� ��
��4��д�����з�Ӧ�Ļ�ѧ����ʽ��
�� ��
�� ��
�� ��
��5��������뺬���ᡢ�Ҵ���ˮ�����������ֲ�Ʒ����ͼ�Ƿ��������������ͼ������ͼ��Բ�����������ʵ����Լ����ڷ������������ʵ��ķ��뷽����
�����Լ�a��__________���Լ�b��_______________��
���뷽������_________�����뷽������____________�����뷽������___________��
�����ڵõ���A�м�����ˮ̼���Ʒ�ĩ����Ŀ����__ ____��
����д��C �� D ��Ӧ�Ļ�ѧ����ʽ ��
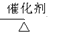 2CH3COOH������AΪ��Ҫԭ�Ϻϳ�������������ϳ�·������ͼ��ʾ��
2CH3COOH������AΪ��Ҫԭ�Ϻϳ�������������ϳ�·������ͼ��ʾ��
�ش��������⣺
��1��д��A�ĵ���ʽ ��
��2��B��D�����еĹ��������Ʒֱ��� �� ��
��3��д�����з�Ӧ�ķ�Ӧ���ͣ��� ���� ���� ��
��4��д�����з�Ӧ�Ļ�ѧ����ʽ��
�� ��
�� ��
�� ��
��5��������뺬���ᡢ�Ҵ���ˮ�����������ֲ�Ʒ����ͼ�Ƿ��������������ͼ������ͼ��Բ�����������ʵ����Լ����ڷ������������ʵ��ķ��뷽����

�����Լ�a��__________���Լ�b��_______________��
���뷽������_________�����뷽������____________�����뷽������___________��
�����ڵõ���A�м�����ˮ̼���Ʒ�ĩ����Ŀ����__ ____��
����д��C �� D ��Ӧ�Ļ�ѧ����ʽ ��
��22�֣���1��  ��2�֣� ��2���ǻ� �� �Ȼ� ����1�֣�
��2�֣� ��2���ǻ� �� �Ȼ� ����1�֣�
��3���ӳɡ� ������ ȡ��������������1�֣�
��4��CH2��CH2 +H2O CH3CH2OH��
CH3CH2OH��
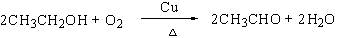
��5��������Na2CO3��Һ��ϡ���
�� ��Һ ���� ���� ���� ���� ������1�֣�
���� ����ˮ�� ���õ��������������� ����2�֣�
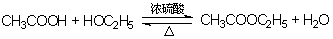
���� 2 CH3COONa + H2SO4 2CH3COOH + Na2SO4 ����2�֣�
2CH3COOH + Na2SO4 ����2�֣�
 ��2�֣� ��2���ǻ� �� �Ȼ� ����1�֣�
��2�֣� ��2���ǻ� �� �Ȼ� ����1�֣���3���ӳɡ� ������ ȡ��������������1�֣�
��4��CH2��CH2 +H2O
 CH3CH2OH��
CH3CH2OH��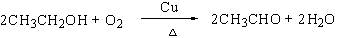

��5��������Na2CO3��Һ��ϡ���
�� ��Һ ���� ���� ���� ���� ������1�֣�
���� ����ˮ�� ���õ��������������� ����2�֣�
���� 2 CH3COONa + H2SO4
 2CH3COOH + Na2SO4 ����2�֣�
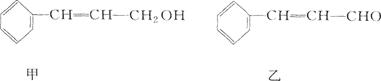
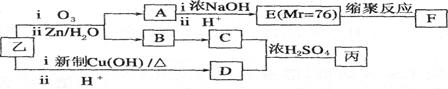
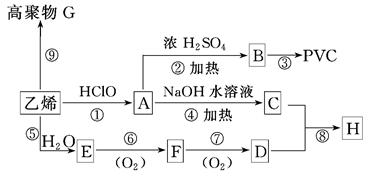
2CH3COOH + Na2SO4 ����2�֣����������A�IJ����Ǻ���һ������ʯ�ͻ�������ˮƽ�ı�־����A����ϩ����ϩ����̼̼˫�����ܺ�ˮ�����ӳɷ�Ӧ�����Ҵ�����B���Ҵ����Ҵ�����������������ȩ����C����ȩ����ȩ�����������ᣬ��D�����ᣬ�Ҵ������ᷢ��������Ӧ���������������������ɵ����������к���������Ҵ������Լ�a�DZ���̼������Һ����Һ����ʵ�����������ķ��룬A���T�õ���������������F������������B�к��������ƺ��Ҵ��Լ�̼���ƣ���ͨ�����õ��Ҵ�������E���Ҵ���C�к��������ƺ�̼���ƣ������ϡ���ἴ�������ᣬȻ������õ����ᡣ
�������������е��Ѷȵ����⣬������۽̲Ļ���֪ʶ��ּ�ڹ���ѧ���Ļ��������ѧ��������û���֪ʶ���ʵ�����������������������ѧ���������������淶��ʵ��������������ѧ����Ӧ������������Ĺؼ�����ͻ�Ƶ��Լ������л���Ľṹ�����ʣ�Ȼ��������ü��ɡ�

��ϰ��ϵ�д�
 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д� ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д�
�����Ŀ
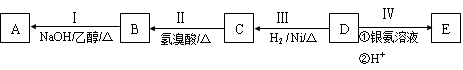
 ��COOCH2CH3��A���١���������Ӧ��C��D��E��B���١���������Ӧ��E��F��H��������Ӧ���̡��������ʼ����ϵ����ͼ��ʾ��
��COOCH2CH3��A���١���������Ӧ��C��D��E��B���١���������Ӧ��E��F��H��������Ӧ���̡��������ʼ����ϵ����ͼ��ʾ��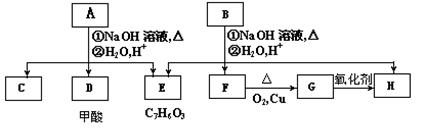
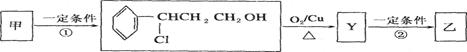
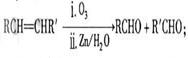
 ��
��
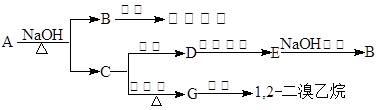
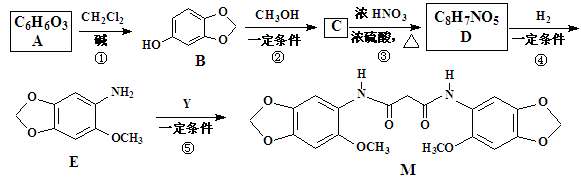
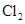 �ֱ���Fe���������������������ʵ���֮��Ϊl��1��Ӧʱ���ֱ��DZ�����һ��ȡ�������ֺͲ�����һ��ȡ����һ�֣�A��
�ֱ���Fe���������������������ʵ���֮��Ϊl��1��Ӧʱ���ֱ��DZ�����һ��ȡ�������ֺͲ�����һ��ȡ����һ�֣�A�� ��Һ��Ӧʱ������ų���A�����з�Ӧ��������B��G��
��Һ��Ӧʱ������ų���A�����з�Ӧ��������B��G��
 ������
������