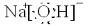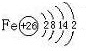题目内容
化学几乎渗透于所有的行业,在考古工作中,化学就起着不可替代的作用。
(1)古墓中,石灰岩(主要成分CaCO3)上绘制的壁画在潮湿的条件下往往覆盖了一层白色薄膜,经测定为CaCO3,用化学反应方程式表示生成CaCO3的过程:
(2)古画颜料中的铅白[Pb2(OH)2CO3]由于受空气中硫化氢气体的作用而变成溶解度极小的黑色硫化铅(反应I),影响画面的色泽,当用过氧化氢处理后,可使硫化铅变成白色的物质(反应Ⅱ)。据此回答下列问题:
①铅白中铅元素的化合价呈 价。
②反应Ⅱ中生成的白色物质的化学式为 。
③反应I的化学反应方程式 。
(3)古代铁器(埋藏在地下)在严重缺氧的环境中,仍然锈蚀严重(电化腐蚀)。原因是一种叫做硫酸盐还原菌的细菌,能提供正极反应的催化剂,可将土壤中的SO42-还原为S2-,该反应放出的能量供给细菌生长、繁殖之需。
①写出该电化腐蚀的正极反应的电极反应 。
②文物出土前,铁器表面的腐蚀产物可能有(写化学式) 。
(1)CaCO3+CO2+H1O=Ca(HCO3)2 Ca(HCO3)2=CaCO3+CO2↑+H2O
(2)+2 PbSO4 Pb2(OH)2CO3+2H2S=2PbS+CO2+3H2O
(3)SO42-+4H2O+8e-=S2-+8OH- Fe(OH)2、FeS