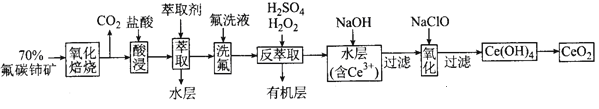
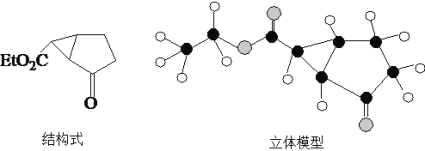
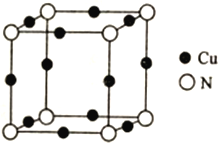
��Ŀ����
����Ŀ������һ������ˮ��Һ�����ܺ������������еļ��֣�![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��ȡ���ݸ�100mL��Һ��������ʵ�飺 ��һ�ݼ���
��ȡ���ݸ�100mL��Һ��������ʵ�飺 ��һ�ݼ���![]() ��Һ�г����������ڶ��ݼ�����NaOH��Һ���Ⱥ��ռ���
��Һ�г����������ڶ��ݼ�����NaOH��Һ���Ⱥ��ռ���![]() ���壻�����ݼ�����
���壻�����ݼ�����![]() ��Һ�õ��������
��Һ�õ��������![]() ������������ϴ�ӡ������������Ϊ
������������ϴ�ӡ������������Ϊ![]() ������Ϊ���½�����ȷ����
������Ϊ���½�����ȷ����
A. �û��Һ��һ�����У�![]() ��
��![]() ��
��![]() ��
��![]() �����ܺ�
�����ܺ�![]() ����
����![]()
B. �û��Һ��һ�����У�![]() ��
��![]() ��
��![]() �����ܺ�
�����ܺ�![]() ��
��![]()
C. �û��Һ��һ�����У�![]() ��
��![]() ��
��![]() �����ܺ�
�����ܺ�![]() ��
��![]() ��
��![]()
D. �û��Һ��һ�����У�![]() ��
��![]() �����ܺ�
�����ܺ�![]() ��
��![]() ��Cl��
��Cl��
���𰸡�A
��������
�������⣬Ba2+��SO42-�ɷ������ӷ�Ӧ����BaSO4������������߲��ܴ������档Ba2+��CO32-�ɷ������ӷ�Ӧ����BaCO3�������������Ҳ���ܴ������棻��һ�ݼ���AgNO3��Һ�г������������ܷ���Cl-+Ag+��AgCl����CO32-+2Ag+��Ag2CO3����SO42-+2Ag+��Ag2SO4�������Կ��ܺ���Cl-��CO32-��SO42-�е�����һ�֣�
�ڶ��ݼ�����NaOH��Һ���Ⱥ��ռ�������0.08mol���ܺ�NaOH��Һ���Ȳ��������ֻ����NH4+�����ݷ�ӦNH4++OH-![]() NH3��+H2O������NH3Ϊ0.08mol���ɵ�NH4+ҲΪ0.08mol��
NH3��+H2O������NH3Ϊ0.08mol���ɵ�NH4+ҲΪ0.08mol��
�����ݼ�����BaCl2��Һ�ø������12.54g������������ϴ�ӣ������������Ϊ4.66g�����ֳ�����������ΪBaCO3�����ֳ�������������ΪBaSO4��������ӦCO32-+Ba2+��BaCO3����SO42-+Ba2+��BaSO4������ΪBaCO3+2HCl��BaCl2+CO2��+H2O��ʹBaCO3�ܽ⣮�����Һ��һ������CO32-��SO42-��һ��������Ba2+��Mg2+����������֪BaSO4Ϊ4.66g�����ʵ���Ϊ0.02mol��BaCO3Ϊ12.54g-4.66g��7.88g�����ʵ���Ϊ0.04mol����CO32-���ʵ���Ϊ0.04mol�������������ɵã���Һ��һ������CO32-��SO42-��NH4+��һ��������Mg2+��Ba2+����CO32-��SO42-��NH4+���ʵ����ֱ�Ϊ0.04mol��0.02mol��0.08mol��CO32-��SO42-��������ɷֱ�Ϊ0.04mol��2��0.02mol��2����0.12mol��NH4+���������Ϊ0.08mol��
A��������Һ�е���غ㣬��֪K+һ�����ڣ�K+���ʵ�����0.04mol����K+���ʵ���=0.04molʱ��û�������ӣ���A��ȷ��
B��������Һ�е���غ㣬��֪K+һ�����ڣ���B����
C����Һ��һ������CO32-��SO42-��NH4+��Mg2+��CO32-�ɷ������ӷ�Ӧ����MgCO3���������棬���Mg2+һ�������ڣ���C����
D����Һ��һ������CO32-��SO42-��NH4+��Mg2+��CO32-�ɷ������ӷ�Ӧ����MgCO3���������棬���Mg2+һ�������ڣ���D����
�𰸣�A

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�