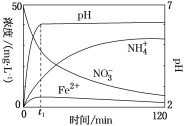
��Ŀ����
����Ŀ��X��Y��Z��M��W���ֶ�����Ԫ�ء�Xԭ�ӵ�����������Ӳ�����ͬ��Wԭ�Ӻ����������Mԭ��������������2����Y��Z��M��W�����ڱ��е����λ����ͼ��ʾ������˵������ȷ����

A. ԭ�Ӱ뾶��W>Y>Z>M>X
B. ���ȶ��ԣ�XM>X2Z
C. ����X��Y��Z����Ԫ���γɵĻ������в����ܺ����Ӽ�
D. WM4������ÿ��ԭ������������8���ӽṹ
���𰸡�C
��������
X��Y��Z��M��WΪ���ֶ�����Ԫ�أ�X��������������Ӳ�����ͬ����XΪHԪ�أ�Y��Z��Mͬ���������ڣ�Ӧ��Ϊ�ڶ�����Ԫ����Wλ�ڵ������ڣ�Wԭ�Ӻ����������Mԭ��������������2������W����������Ϊx����2+8+x=2(x+3)�����x=4����WΪSi��YΪNԪ�ء�ZΪOԪ�أ�MΪFԪ�أ��ݴ˷������
��������������XΪHԪ�ء�YΪNԪ����ZΪOԪ�ء�MΪFԪ�ء�WΪSiԪ����A��ͬ�����������ԭ�Ӱ뾶��С�����Ӳ�Խ��ԭ�Ӱ뾶Խ��ԭ�Ӱ뾶Si��N��O��F��H����A��ȷ��B��XMΪHF��X2ZΪH2O��Ԫ�صķǽ�����Խǿ������̬�⻯��Խ�ȶ������ڷǽ�����F��O�������ȶ���HF��H2O����B��ȷ��C��H��N��O����Ԫ���γɵĻ��������������泥�������м������Ӽ����й��ۼ�����C����D��WM4ΪSiF4��Si��Fԭ������������8���ӽṹ����D��ȷ����ѡC��

 ������ѧ���̲���ȫ���ϵ�д�
������ѧ���̲���ȫ���ϵ�д�����Ŀ��������Ҫ�Ļ�������ԭ�ϣ������Ʊ�������(HNO2)������������(H2N2O2)������[CO(NH2)2]�ȶ��ֺ����Ļ�����Ʒ
��1����֪��25��ʱ�������������������ĵ��볣�����±���ʾ��
��ѧʽ | HNO2 | H2N2O2 |
���볣�� | Ka=5.1��104 | Ka1=6.17��108 ��Ka2=2.88��1012 |
�����ʵ���Ũ����ͬ��NaNO2��NaHN2O2��Һ��pH(NaNO2)_________pH��NaHN2O2)���>����<����=������
��25��ʱ��NaHN2O2��Һ�д���ˮ��ƽ�⣬��ˮ�ⳣ��Kh=____��������λ��Ч���֣���
��0.1mol/L NaHN2O2��Һ������Ũ���ɴ�СΪ��________________________________
��2����NH3��CO2Ϊԭ�Ͽ��Ժϳ�����[CO(NH2)2]���漰�Ļ�ѧ��Ӧ���£�
��ӦI��2NH3(g)+CO2(g)![]() NH2CO2NH4(s) ��H1=159.5 kJ��mol1��
NH2CO2NH4(s) ��H1=159.5 kJ��mol1��
��ӦII��NH2CO2NH4(s)![]() CO(NH2)2(s)+H2O(g) ��H2=+116.5 kJ��mol1��
CO(NH2)2(s)+H2O(g) ��H2=+116.5 kJ��mol1��
��ӦIII��H2O(l)![]() H2O(g) ��H3=+44.0 kJ��mol1��
H2O(g) ��H3=+44.0 kJ��mol1��
��ӦIV��NH3��CO2�ϳ�����ͬʱ����Һ̬ˮ���Ȼ�ѧ����ʽΪ__________________��
��3��T1��ʱ�����ݻ�Ϊ2 L�ĺ����ܱ������г���n(NH3)��n(CO2)=2��1��ԭ������ʹ֮������ӦIV����Ӧ������õ����ص�����Ϊ30 g�������ڵ�ѹǿ(p)��ʱ��(t)�ı仯��ͼ1��ʾ��
��T1��ʱ���÷�Ӧ��ƽ�ⳣ��K��ֵΪ______________��
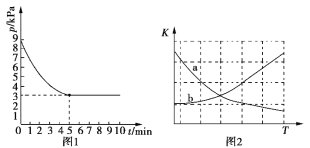
��ͼ2������ȷ��ӳƽ�ⳣ��K���¶ȱ仯��ϵ������Ϊ________�������߱����ĸ����