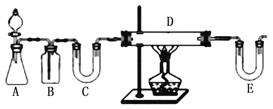
��Ŀ����
����Ŀ��Ϊ��������̼������СCO2�Ի�����Ӱ�죬���CO2���д��������о���T1��ʹ������9 mol CO2��12 mol H2����3 L�ܱ�������,������ӦCO2(g)+3H2(g) ![]() CH3OH(g)+H2O(g) ��H��0��������CH3OH�����ʵ�����ʱ��仯�����ߢ���ʾ,ƽ��ʱ������ѹǿΪp0���ı�ijһ�������½���������Ӧ��CH3OH�����ʵ�����ʱ��仯�����ߢ���ʾ������˵���������
CH3OH(g)+H2O(g) ��H��0��������CH3OH�����ʵ�����ʱ��仯�����ߢ���ʾ,ƽ��ʱ������ѹǿΪp0���ı�ijһ�������½���������Ӧ��CH3OH�����ʵ�����ʱ��仯�����ߢ���ʾ������˵���������
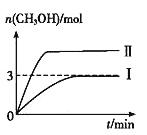
A. ���ߢ��Ӧ�������ı�������ѹǿ
B. T2��ʱ,������Ӧƽ�ⳣ��Ϊ0.52����T2>T1
C. ��T1�棬����ʼʱ����������4.5 mol CO2��6 mol H2��ƽ��ʱ������ѹǿP1>P0/2
D. ��T1��,����ʼʱ�������г���4.5 mol CO2��5 mol H2��5 mol CH3OH(g)��5 mol H2O(g)�����ƽ��ǰv(��)>v(��)
���𰸡�B
��������A������ѹǿ����ѧ��Ӧ�������ﵽƽ������ʱ��϶̣�����ѹǿ��ƽ��������Ӧ������У��״������ʵ�������A˵����ȷ��B��
CO2(g)+3H2(g) ![]() CH3OH(g)+H2O(g)
CH3OH(g)+H2O(g)
��ʼ�� 9 12 0 0
�仯�� 3 9 3 3
ƽ�⣺ 6 3 3 3 ���ݻ�ѧƽ�ⳣ���ı���ʽ��K=c(CH3OH)��c(H2O)/[c3(H2)��c(CO2)]=(1��1)/(13��2)=0.5�� 0.5<0.52��˵����Ӧ��������Ӧ������У��˷�Ӧ�Ƿ��ȷ�Ӧ�������¶�ƽ�����淴Ӧ������У���T2<T1����B˵������C����9molCO2��12molH2�Ļ����ϼ���һ�룬����ƽ�ⲻ�ƶ�����ʱ��ѹǿ��ԭ����һ�룬��Ӧ���Ũ�ȼ��٣�ƽ�����淴Ӧ�����ƶ����������ʵ�������P1>P0/2����C˵����ȷ��D����ʱ��Ũ����Qc= 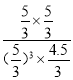 =0.4<0.5��˵����Ӧ������Ӧ�����ƶ�����v(��)>v(��) ����D˵����ȷ��
=0.4<0.5��˵����Ӧ������Ӧ�����ƶ�����v(��)>v(��) ����D˵����ȷ��

 һ����������ϵ�д�
һ����������ϵ�д�����Ŀ��ú������Ҫ�ĺ���������H2S�Լ�COS���л���ú��ȼ�պ������ʻ�ת����SO2�Ӷ����������Ⱦ��ú����H2S���ѳ��̶��ѳ�Ϊ��ྻ�ȵ�һ����Ҫָ�ꡣ�ش��������⣺
(1)��H2Sͨ��FeCl3��Һ�У��÷�Ӧ�Ļ�ԭ����Ϊ___________��
(2)�ѳ�ú����COS�ķ�����Br2��KOH��Һ��������H2��ԭ���Լ�ˮ�ⷨ�ȡ�
��COS�ķ��ӽṹ��CO2���ƣ�COS�ĵ���ʽΪ_____________��
��Br2��KOH��Һ��COS����Ϊ�����κ�̼���ε����ӷ���ʽΪ_____________��
����֪����1mol��ѧ��������������£�
��ѧ�� | H��H | C�TO | C�TS | H��S | C��O |
E/kJmol-1 | 436 | 745 | 577 | 339 | 1072 |
H2��ԭCOS�����ķ�ӦΪH2(g)+COS(g) �TH2S(g)+CO(g)���÷�Ӧ�Ħ�H=________kJ��mol-1��
���û��Ԧ���Al2O3��COSˮ��ķ�ӦΪCOS(g)+ H2 O(g) ![]() CO2(g)+ H2S (g) ��H<0����ͬͶ�ϱȡ���ͬ�������ڴ�������ͣ����ͬʱ��ʱ����ͬ�¶���COS��ת���ʣ�δ�ﵽƽ�⣩��ͼ1��ʾ��ij�¶��£�COS��ƽ��ת������
CO2(g)+ H2S (g) ��H<0����ͬͶ�ϱȡ���ͬ�������ڴ�������ͣ����ͬʱ��ʱ����ͬ�¶���COS��ת���ʣ�δ�ﵽƽ�⣩��ͼ1��ʾ��ij�¶��£�COS��ƽ��ת������![]() �Ĺ�ϵ��ͼ2��ʾ��
�Ĺ�ϵ��ͼ2��ʾ��
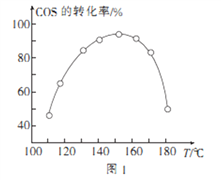

��ͼ1��֪�������������ʱ��Ӧ���¶�ԼΪ____________����ͼ2��֪��P��ʱƽ�ⳣ��K=_____
������2λ��Ч���֣���
(3)�����¿ɷ�����Ӧ��ZnS(s)+2H+(aq) ![]() Zn2+(aq)+H2S(aq)���ﵽƽ��ʱ���÷�Ӧ��ƽ�ⳣ��K=_______[��֪��Ksp(ZnS)=2.93��10-25��H2S���볣����Ka1=9.5��10-8��Ka2=1.3��10-14]��
Zn2+(aq)+H2S(aq)���ﵽƽ��ʱ���÷�Ӧ��ƽ�ⳣ��K=_______[��֪��Ksp(ZnS)=2.93��10-25��H2S���볣����Ka1=9.5��10-8��Ka2=1.3��10-14]��