��Ŀ����
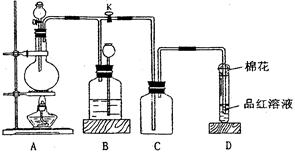
��14�֣������״�(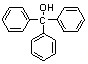 )����Ҫ���л��ϳ��м��壬ʵ�����кϳ������״�ʱ����ͼ2��ʾ��װ�ã���ϳ�������ͼ1��ʾ��
)����Ҫ���л��ϳ��м��壬ʵ�����кϳ������״�ʱ����ͼ2��ʾ��װ�ã���ϳ�������ͼ1��ʾ��

ͼ1
��֪����1�� ����ʽ�廯þ����
����ʽ�廯þ����
��2��������ʵ������������£�
��ش��������⣺
��1��д��װ��ͼ�в������������ƣ�A ������B������ ��
��2����ȡ�����Լ�ʱҪ�����У����Բ���ˮԡ���ȣ��ŵ��� ���л���ʱ��������ˮ���ķ�����
���X��Y����Y��X������װ����ˮCaCl2������A�������� ��
��3���Ƶõ������״��ֲ�Ʒ�У��������ѡ��屽���������������л���ͼ�ʽ�廯þ�����ʣ�������������ᴿ����������д�հף�


ͼ2 ͼ3
��4������ʱ����װ����ͼ3��ʾ����װ�ô��ڵĴ����� ��������ϻ���;ֹͣ����ʱ�IJ���������Ӧ�� ��Ȼ�� ��
��5��ϴ��Һ���ѡ�� �������Ʒ�Ѿ�ϴ�Ӹɾ��IJ���Ϊ ��
��A��ˮ��B������ ��C���Ҵ� ��D����
 )����Ҫ���л��ϳ��м��壬ʵ�����кϳ������״�ʱ����ͼ2��ʾ��װ�ã���ϳ�������ͼ1��ʾ��
)����Ҫ���л��ϳ��м��壬ʵ�����кϳ������״�ʱ����ͼ2��ʾ��װ�ã���ϳ�������ͼ1��ʾ��
ͼ1
��֪����1��
 ����ʽ�廯þ����
����ʽ�廯þ������2��������ʵ������������£�
| ���� | �۵� | �е� | �ܽ��� |
| �����״� | 164.2�� | 380�� | ������ˮ�������Ҵ������ѵ��л��ܼ� |
| ���� | -116.3�� | 34.6�� | ����ˮ�������Ҵ��������л��ܼ� |
| �屽 | -30.7��C | 156.2��C | ������ˮ�������Ҵ������ѵȶ����л��ܼ� |
| ���������� | -34.6��C | 212.6��C | ������ˮ |
| Mg(OH)Br | ������Ϊ���� | ������ˮ�������ڴ����ѵ��л��ܼ� | |
��1��д��װ��ͼ�в������������ƣ�A ������B������ ��
��2����ȡ�����Լ�ʱҪ�����У����Բ���ˮԡ���ȣ��ŵ��� ���л���ʱ��������ˮ���ķ�����
���X��Y����Y��X������װ����ˮCaCl2������A�������� ��
��3���Ƶõ������״��ֲ�Ʒ�У��������ѡ��屽���������������л���ͼ�ʽ�廯þ�����ʣ�������������ᴿ����������д�հף�



ͼ2 ͼ3
��4������ʱ����װ����ͼ3��ʾ����װ�ô��ڵĴ����� ��������ϻ���;ֹͣ����ʱ�IJ���������Ӧ�� ��Ȼ�� ��
��5��ϴ��Һ���ѡ�� �������Ʒ�Ѿ�ϴ�Ӹɾ��IJ���Ϊ ��
��A��ˮ��B������ ��C���Ҵ� ��D����
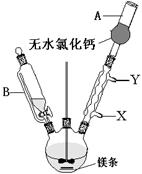
��1�������[1��],ƽ��ѹǿ[1��]��������©������ѹǿ��ͬʹ�Լ����ڵ��¡��ȣ�
��2�����Ⱦ���[1��]���¶����ڿ���[1��]�� ��X��Y��[1��]��
��ֹ������ˮ��������װ��[1��]ʹ�����Լ�ˮ��
��3������[1��]
��4������©������б��δ�����ƿ֧�ܿ����[1��]����ȫƿ�г��ܺͶ̹ܵ�����˳��Ū��[1��]
�Ͽ�����ƿ�밲ȫƿ�����ӣ���ȫƿ��ˮ��ͷ�����ӣ�[1��]���ر�ˮ��ͷ[1��]��
��5��A[1��]��ȡ���һ�ε�ϴ��Һ[1��]���μ�NaOH��Һ����AgNO3��Һ��[1��]����ϴ��Һδ�����ǣ�����ϴ�Ӹɾ����μӷ�̪��Һ��ϴ��Һδ��ɫ���ȣ���
��2�����Ⱦ���[1��]���¶����ڿ���[1��]�� ��X��Y��[1��]��
��ֹ������ˮ��������װ��[1��]ʹ�����Լ�ˮ��
��3������[1��]
��4������©������б��δ�����ƿ֧�ܿ����[1��]����ȫƿ�г��ܺͶ̹ܵ�����˳��Ū��[1��]
�Ͽ�����ƿ�밲ȫƿ�����ӣ���ȫƿ��ˮ��ͷ�����ӣ�[1��]���ر�ˮ��ͷ[1��]��
��5��A[1��]��ȡ���һ�ε�ϴ��Һ[1��]���μ�NaOH��Һ����AgNO3��Һ��[1��]����ϴ��Һδ�����ǣ�����ϴ�Ӹɾ����μӷ�̪��Һ��ϴ��Һδ��ɫ���ȣ���
��1�����������Ľṹ�ص��֪��A�Ǹ���ܣ�����B�Ľṹ��֪����Ҫ�����DZ���©������ѹǿ��ͬʹ�Լ����ڵ��¡�
��2��ˮԡ���ȿ���ʹ��Һ���Ⱦ��ȣ����¶����ڿ��ƣ���ȴˮ����������������Ӧ�����෴�ģ����Դ��Ǵ�X��Y���������⣬�����Լ���ˮ�⣬�������к���ˮ���������������Ƿ�ֹ������ˮ��������װ�ã�ʹ�����Լ�ˮ�⡣
��3���������ʵ��������ʿ�֪�����ǵķе����ϴ����Է���ķ���������
��4������ͼ3��֪�����ڵĴ����Dz���©������б��δ�����ƿ֧�ܿ���ԣ�����ǰ�ȫƿ�г��ܺͶ̹ܵ�����˳��Ū����ʵ�������Ӧ���ȶϿ�����ƿ�밲ȫƿ�����ӣ���ȫƿ��ˮ��ͷ�����ӣ���Ȼ���ٹر�ˮ��ͷ��
��5�����������״��������л��ܼ��У���������ˮ�����Ӧ����ˮϴ�ӣ���ѡA������ϴ��Һ�к��������ӣ��ݴ˿��ü��𣬼�ȡ���һ�ε�ϴ��Һ���μ�NaOH��Һ����AgNO3��Һ������ϴ��Һδ�����ǣ�����ϴ�Ӹɾ���
��2��ˮԡ���ȿ���ʹ��Һ���Ⱦ��ȣ����¶����ڿ��ƣ���ȴˮ����������������Ӧ�����෴�ģ����Դ��Ǵ�X��Y���������⣬�����Լ���ˮ�⣬�������к���ˮ���������������Ƿ�ֹ������ˮ��������װ�ã�ʹ�����Լ�ˮ�⡣
��3���������ʵ��������ʿ�֪�����ǵķе����ϴ����Է���ķ���������
��4������ͼ3��֪�����ڵĴ����Dz���©������б��δ�����ƿ֧�ܿ���ԣ�����ǰ�ȫƿ�г��ܺͶ̹ܵ�����˳��Ū����ʵ�������Ӧ���ȶϿ�����ƿ�밲ȫƿ�����ӣ���ȫƿ��ˮ��ͷ�����ӣ���Ȼ���ٹر�ˮ��ͷ��
��5�����������״��������л��ܼ��У���������ˮ�����Ӧ����ˮϴ�ӣ���ѡA������ϴ��Һ�к��������ӣ��ݴ˿��ü��𣬼�ȡ���һ�ε�ϴ��Һ���μ�NaOH��Һ����AgNO3��Һ������ϴ��Һδ�����ǣ�����ϴ�Ӹɾ���

��ϰ��ϵ�д�
�����Ŀ