��Ŀ����
1����ˮAlCl3���������������л��ϳɵĴ����ȣ���ҵ����������A12O3��Fe2O3��Ϊԭ���Ʊ���ˮAlCl3�Ĺ����������£�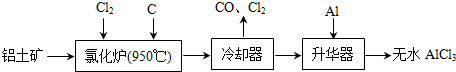
��1���Ȼ�¯��Al2O3��Cl2��C��Ӧ�Ļ�ѧ����ʽΪA12O3+3C12+3C$\frac{\underline{\;950��\;}}{\;}$2A1C13+3CO��
��2����Na2SO3��Һ�ɳ�ȥ��ȴ���ų�β���е�Cl2���˷�Ӧ�����ӷ���ʽΪSO32-+C12+H2O=SO42-+2C1-+2H+���ڱ�״���£�����112L Cl2��Ҫ5mol Na2SO3��
��3��Ϊ�ⶨ�Ƶõ���ˮAlCl3��Ʒ��������FeCl3���Ĵ��ȣ���ȡ��ˮAlCl3��Ʒ�����ڹ�����NaOH��Һ�����˳��������ϴ�ӡ����ա���ȴ�����أ���д���ⶨ��ˮAlCl3��Ʒ���ȵĹ������йط�Ӧ�����ӷ���ʽ��
Fe3++3OH-=Fe��OH��3����Al3++4OH-=AlO2-+2H2O��
��4����ҵ����һ��������Ϊԭ���Ʊ���ˮAlCl3�����У����һ������AlCl3•6H2O��ȥ�ᾧˮ�Ʊ���ˮAlCl3��ʵ����һ���IJ����������ڸ���HCl�����м��ȣ�
��5����ҵ���������ᴿ���ұ������д����950-970���Na3AlF6�����½��е��������Ӧ�Ļ�ѧ����ʽ2A12O3$\frac{\underline{\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;���\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;}}{950��--970��Na_{3}AlF_{6}}$4Al+3O2����
��6��Ϊ�ⶨ�Ƶõ���ˮAlCl3��Ʒ��������FeCl3���Ĵ��ȣ���ȡ16.25����ˮAlCl3��Ʒ���ڹ�����NaOH��Һ�����˳�����������ᆳϴ�ӡ����ա���ȴ�����أ�������������Ϊ0.32�ˣ���AlCl3��Ʒ�Ĵ���Ϊ96%��
���� ���ݹ������̿�֪�Ȼ�¯�IJ������ȴ���������Ʊ���ˮAlCl3��˵���Ȼ�¯�IJ����к���A1C13��������β������CO������Al2O3��C12��C��Ӧ������A1C13��CO��A1C13Ϊ���ۻ��������������Ҫ����AlCl3��FeCl3��FeCl3�۵㡢�е�ϸߣ������Ʊ���ˮAlCl3����������AlĿ���dz�ȥFeCl3��
��1�����ݹ������̿�֪�������к���AlCl3�ȣ�����Al2O3��C12��C��Ӧ������A1C13����������β����֪��������CO��
��2��Cl2��ǿ�����ԣ���SO32-����ΪSO42-����������ԭΪC1-����Ϸ�Ӧ�ķ���ʽ��������Na2SO3�����ʵ�����
��3�����ӹ����������������������ӽ�ϳ��������������������������������ӽ�ϳ�ƫ�������ˮ��
��4���Ȼ�����Һ����ˮ�⣬��AlCl3•6H2O��ˮ�Ʊ���ˮ�Ȼ�������HCl�����м����ѽᾧˮ��
��5����������950-970���Na3AlF6�����½��е����������������
��6���Ʊ���ˮAlCl3��������FeCl3��������������Ϊ0.32gΪFe2O3��������ԭ���غ����FeCl3���������������AlCl3���������ٸ��ݲ�Ʒ�Ĵ��ȶ�����㣮
��� �⣺��1�����ݹ������̿�֪�Ȼ�¯�IJ������ȴ���������Ʊ���ˮAlCl3��˵���Ȼ�¯�IJ����к���A1C13��������β������CO������Al2O3��C12��C��Ӧ������A1C13��CO����Ӧ����ʽΪA12O3+3C12+3C$\frac{\underline{\;950��\;}}{\;}$2A1C13+3CO��
�ʴ�Ϊ��A12O3+3C12+3C$\frac{\underline{\;950��\;}}{\;}$2A1C13+3CO��
��2��Cl2��ǿ�����ԣ���SO32-����ΪSO42-����������ԭΪ2C1-����Ӧ���ӷ���ʽΪSO32-+C12+H2O�TSO42-+2C1-+2H+��
�ڱ�״���£�112L Cl2�����ʵ���Ϊ5mol�����ɷ���ʽ��֪Ӧ����5molNa2SO3��
�ʴ�Ϊ��SO32-+C12+H2O=SO42-+2C1-+2H+��5��
��3�����ӹ����������������������ӽ�ϳ��������������������������������ӽ�ϳ�ƫ�������ˮ��
���ӷ���ʽΪ��Fe3++3OH-=Fe��OH��3����Al3++4OH-=AlO2-+2H2O��
�ʴ�Ϊ��Fe3++3OH-=Fe��OH��3����Al3++4OH-=AlO2-+2H2O��
��4���Ȼ�����Һ����ˮ�⣬��AlCl3•6H2O��ˮ�Ʊ���ˮ�Ȼ�������HCl�����м����ѽᾧˮ��
�ʴ�Ϊ���ڸ���HCl�����м��ȣ�
��5����������950-970���Na3AlF6�����½��е��������������������ʽΪ2A12O3$\frac{\underline{\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;���\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;}}{950��--970��Na_{3}AlF_{6}}$4Al+3O2����
�ʴ�Ϊ��2A12O3$\frac{\underline{\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;���\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;}}{950��--970��Na_{3}AlF_{6}}$4Al+3O2����
��6��������ԭ���غ㣬�������FeCl3����Ϊmg����
Fe2O3����������2FeCl3
160 325
0.32g mg
���� $\frac{0.32g}{160}$=$\frac{mg}{325}$����ã�m=0.65g��
����AlCl3��Ʒ�Ĵ���Ϊ$\frac{16.25g-0.65g}{16.25g}$��100%=96%��
�ʴ�Ϊ��96%��
���� ���⿼���Ʊ���ˮAlCl3�Ĺ�������ԭ�������⡢�Բ�����ʵ���������Ƶ�����ȣ��漰���û�ѧ������д�����㡢�����ᴿ�ȣ���Ҫѧ���߱���ʵ�Ļ������ۺ�������������Ŀ�Ѷ��еȣ�

 �ƸԴ��ž�ϵ�д�
�ƸԴ��ž�ϵ�д�| A�� | ��K37ClO3��Ũ������������37ClO3-+6H++6Cl-�T37Cl-+3Cl2��+3H2O | |
| B�� | ����Һ�е����ʵ�����FeBr2��Cl2��Ӧ��2Fe2++2Br-+2Cl2�T2Fe3++Br2+4Cl- | |
| C�� | ��1mol/L��NaAlO2��Һ��2.5mol/L��HCl��Һ��������Ȼ�� AlO2-+H++H2O�TAl��OH��3�� | |
| D�� | 0.1mol/L��Na2CO3��Һ��2 C��Na+���TC ��HCO3-��+C ��H2CO3��+C��CO32-�� |
| A�� | W��X������������Ӧˮ��������ԣ�X��W | |
| B�� | ���ʷе㣺Y��Z��W | |
| C�� | ���Ӱ뾶��X-��W-��Y+��Z- | |
| D�� | �������������X2-��W- |
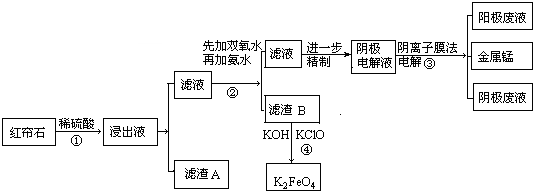
��1����ҵ��Ϊ���ϡ�����ȡЧ��һ���ȡ�Ĵ�ʩ�ǣ�����д���ַ�����
�ٷ������ʯ����߷�Ӧ���¶ȣ�
��2������Һ�е���������H+��Fe2+��Fe3+��Mg2+��Mn2+���������ӷ��ţ�
��3������A����MnO2ԭ��MnO2�����������±���������ԭΪMn2+��
��4����֪��ͬ����������������������������pH���±���
| ���� | Fe3+ | Fe2+ | Mn2+ | Mg2+ |
| ��ʼ������pH | 2.7 | 7.0 | 7.8 | 9.3 |
| ��ȫ������pH | 3.7 | 9.6 | 9.8 | 10.8 |
���̢��мӰ�ˮ������Һ��pH����6��������B�ijɷ�Fe��OH��3��
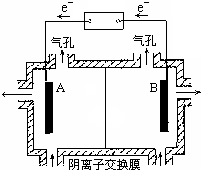
��5�����װ����ͼ��ʾ������Һ���������ƶ��ķ���ΪA��B���A��B����B��A������ʵ�������У�������ϡ����Ϊ���Һ�������ĵ缫��ӦʽΪ2H2O-4e-=O2��+4H+��
��6������B����Ӧ�����ɸ�Чˮ�����������ӷ���ʽ2Fe��OH��3+3ClO-+4OH-=2FeO42-+3Cl-+5H2O��K2FeO4����Ϊ��Чˮ��������ԭ����FeO42-����ǿ�����Կ���ɱ����������ԭ��Fe3+ˮ���Fe��OH��3�����������������ɳ�����

�����ʵ�鲽�裬�ش��������⣺
��1���ر�a��b����ͨ��ֱ�����ܵ�����ˮ����A����30���ӣ��Ʊ�1-�嶡�飮��ֱ�����ܽ�ͨ����ˮ����ˮ���Ǣ��I������������������ҪĿ���dz�ַ�Ӧ����߷�Ӧ���ת���ʣ�
��2�������ϣ�������Ӧ�ĸ���������У����ѣ�CH3CH2CH2CH2-O-CH2CH2CH2CH3����1-��ϩ���廯�⡢�������ơ�ˮ�ȣ�Ϩ��ƾ��ƣ�����ֱ�������Ϸ��������Ӳ���a���������ȼ�����Ӧֱ����ȴ��ͨ��B��Cװ�ü��鲿�ָ����B��C��Ӧʢ�ŵ��Լ��ֱ���ʯ����Һ����AgNO3��Һ������ˮ��������KMnO4��Һ����д��Cװ������Ҫ�Ļ�ѧ����ʽ��CH3CH2CH=CH2+Br2��BrCH2CHBrCH2CH3 ��
��3��Ϊ�˽�һ�������ᴿ1-�嶡�飬����ȤС��ͬѧ�������л�������������ʾ��
| ���� | �۵�/�� | �е�/�� |
| 1-���� | -89.5 | 117.3 |
| 1-�嶡�� | -112.4 | 101.6 |
| ���� | -95.3 | 142.4 |
| 1-��ϩ | -185.3 | -6.5 |
�ٴ���ƿ��ȴ��ȥ��ֱ�������ܣ�
�ڲ��ϴ���Ƥ�����¶ȼƣ�
�۹ر�a����b��
�ܽ�ͨ�����ܵ�����ˮ��ʹ��ˮ��d�����룻
��Ѹ�������¶���101.6�棬�ռ�������֣�
��4����ʵ������ȡ1-������NaBr�ֱ�Ϊ7.4g��13.0g�������Ĵֲ��ᆳϴ�ӡ�������ٴ�����õ�9.6g 1-�嶡�飬��1-�嶡��IJ�����70%��������2λ��Ч���֣�
| A�� |  | B�� |  | C�� |  | D�� |  |