��Ŀ����
����Ŀ���о����ʵĽṹ������̽Ѱ���ʵ����ʣ�������ѧϰ��ѧ����Ҫ�������ش��������⣺
(1)Fe��Ru��Os��Ԫ�����ڱ��д���ͬһ�У������Ѿ����ֺ�Ӧ����Ru��Os������������ӻ�ѧ����Ԥ����Ҳ��������������������Ƿ������Ļ��ϼ۲���+8����+6��OsO4���ӿռ���״��____________�����������������������У����ļ۵����Ų�ʽ��____________�����Ļ��ϼ���___________��
(2)NH3������H��N��H����Ϊ106.7������Ag(NH3)2+�У�H��N��H���ǽ���109.5�������DZ���ԭ����_______________________��
(3)����ı�����ȱ���ӵ���ԭ�Ӻ����ӵ�ԭ�ӻ�ԭ����֮���һ�����ĵ������á������������Ƿ�����˫�����˫�����ָ�������Hԭ����������Hԭ��֮���һ������������á����в������γ�˫�������_______��
a��Be��H��H��O b��O��H��H��N c��B��H��H��N d��Si��H��H��Al
(4)����ʯ(Na3A1F6)��Ҫ������������������ۼ���Ҳ������ĥ��Ʒ����ĥ���Ӽ����侧���ṹ��ͼ��ʾ������������������״��Na(I)λ�ڲ������ĺ͵������ģ�Na(II)λ���ĸ������ϣ�AlF63-λ�ڶ�������ġ�
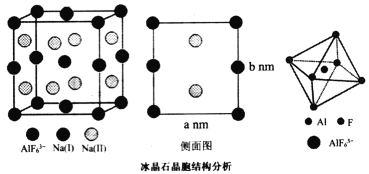
AlF![]() �У�����ԭ����Χ�ijɼ�����������________��������ԭ����������������������Naԭ�ӵ�λ�ã�����Ҫ________��ԭ�����ꡣ��֪�����߳�Ϊa nm��b nm������ʯ������ܶ�Ϊ__________g��cm-3(Na3AlF6��Ħ������Ϊ210g��mol-1)��
�У�����ԭ����Χ�ijɼ�����������________��������ԭ����������������������Naԭ�ӵ�λ�ã�����Ҫ________��ԭ�����ꡣ��֪�����߳�Ϊa nm��b nm������ʯ������ܶ�Ϊ__________g��cm-3(Na3AlF6��Ħ������Ϊ210g��mol-1)��
���𰸡��������� 3d2 -1��-2 [Ag(NH3)2]+�У���λ��N-Ag���ijɼ����Ӷ����NH3�еŵ��Ӷ�N-H�����ų�����С����[Ag(NH3)2]+��H-N-H���DZ�� bd 12 6 ![]()
��������
(1)Fe��Ru��Os��Ԫ�����ڱ��д���ͬһ�У������������ԡ�OsO4���ӿռ���״��FeO4��ͬ�����������壬���������������������У������۵��������3d����ϵ�ֵΪ3+0.4��2=3.8����4s���Ϊ4+0.4��0=4.0��3d<4s���������ļ۵����Ų�ʽ��3d2�����Ļ��ϼ�Ϊ+6�������Ļ��ϼ���-1��-2��
(2)NH3������H��N��H����Ϊ106.7������Ag(NH3)2+�У�H��N��H���ǽ���109.5�������DZ���ԭ����[Ag(NH3)2]+�У���λ��N-Ag���ijɼ����Ӷ����NH3�еŵ��Ӷ�N-H�����ų�����С����[Ag(NH3)2]+��H-N-H���DZ��
(3)����ı�����ȱ���ӵ���ԭ�Ӻ����ӵ�ԭ�ӻ�ԭ����֮���һ�����ĵ������á�˫�����ָ�������Hԭ����������Hԭ��֮���һ������������á�
a��Be��H����ԭ�Ӵ����磬H��O����ԭ�Ӵ����磬����˫������壻
b��O��H��H��N����ԭ�Ӿ������磬���������⣻
c��B��H����ԭ�Ӵ����磬H��N������ԭ�Ӵ����磬����˫������壻
d��Si��H��H��Al����ԭ�Ӿ������磬���������⡣
��Ϊbd��
(4)����ʯ(Na3A1F6)����������������״��Na(I)λ�ڲ������ĺ͵������ģ�Na(II)λ���ĸ������ϣ�AlF63-λ�ڶ�������ġ�
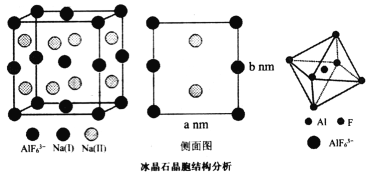
���ݽṹ��֪��AlF![]() �У�����ԭ����Χ�ijɼ�����������12��������ԭ����������������������Naԭ�ӵ�λ�ã�����Ҫ6��ԭ�����ꡣ��֪�����߳�Ϊa nm��b nm������ʯ������ܶ�Ϊ
�У�����ԭ����Χ�ijɼ�����������12��������ԭ����������������������Naԭ�ӵ�λ�ã�����Ҫ6��ԭ�����ꡣ��֪�����߳�Ϊa nm��b nm������ʯ������ܶ�Ϊ![]()
![]() g��cm-3(Na3AlF6��Ħ������Ϊ210g��mol-1)��
g��cm-3(Na3AlF6��Ħ������Ϊ210g��mol-1)��

����Ŀ����������![]() �����ڻ�ѧ������������
�����ڻ�ѧ������������![]() �����ռ���Һ��ϡ����ȣ����ɴ������ƺ�
�����ռ���Һ��ϡ����ȣ����ɴ������ƺ�![]() ��
��![]() ��һ����ɫ���ж��Ŀ�ȼ�����壬��
��һ����ɫ���ж��Ŀ�ȼ�����壬��![]() ��Һ��ӦҲ�����ɴ������ơ�ʵ���ҿ�������װ������ȡ�������ơ�ʵ����йز����������£�
��Һ��ӦҲ�����ɴ������ơ�ʵ���ҿ�������װ������ȡ�������ơ�ʵ����йز����������£�
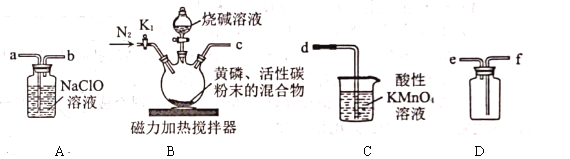
��װ��ҩƷ��������װ�ã���������ԣ��۴�![]() ����ͨ��һ��ʱ��
����ͨ��һ��ʱ��![]() ���ܽ���Ӧ���õĴ������Ʒ���������ݹر�
���ܽ���Ӧ���õĴ������Ʒ���������ݹر�![]() ���μ��ռ���Һ���ٴ������Ƚ���������
���μ��ռ���Һ���ٴ������Ƚ���������![]() ͨ��
ͨ��![]() һ��ʱ�䡣��ش��������⣺
һ��ʱ�䡣��ش��������⣺
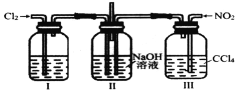
��1����ȷʵ����������˳����________������ţ���
��2��ʵ��װ���и����ܿڵ�����˳����________����װ���е�Сд��ĸ����
��3���ڻ����л������̿��ĩ��������________��
��4���ȵμ��ռ���Һ���ٴ������Ƚ��������ܵ�ԭ����________��
��5��װ��![]() �з�Ӧ�Ļ�ѧ����ʽ��________��
�з�Ӧ�Ļ�ѧ����ʽ��________��
��6��![]() ��
��![]() ���ܽ��
���ܽ��![]() ���£�
���£�
|
| |
| 37 | 39 |
| 100 | 667 |
ʵ�������A�л��Һ����Ũ�����д��������������þ�����Ҫ�ɷֵĻ�ѧʽΪ________��Ȼ��________����ȴ�ᾧ�����ˡ�ϴ�ӡ�����õ���![]() �Ĵֲ�Ʒ��
�Ĵֲ�Ʒ��
��7���ⶨ��Ʒ�Ĵ��ȣ�ȡ��Ʒ![]() �����
�����![]() ��Һ��ȡ
��Һ��ȡ![]() ����ƿ�У��ữ�����
����ƿ�У��ữ�����![]() ��ˮ�ڰ�����ַ�Ӧ��
��ˮ�ڰ�����ַ�Ӧ��![]() ��Ȼ���Ե�����Һ��ָʾ������
��Ȼ���Ե�����Һ��ָʾ������![]() ��Һ�ζ����յ㣬ƽ������
��Һ�ζ����յ㣬ƽ������![]() �����������ʲ��μӷ�Ӧ�����Ʒ���ȱ���ʽΪ________������֪��
�����������ʲ��μӷ�Ӧ�����Ʒ���ȱ���ʽΪ________������֪��![]() ��
��