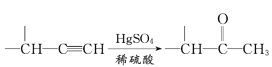
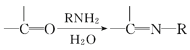
��Ŀ����
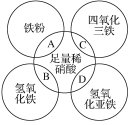
����Ŀ��ij�������������Fe2O3��Fe��Na2SO3��NaBr��AgNO3��BaCl2�е����ֻ��������ϵ�������ɡ�ij��ȤС��Ϊ̽���ù����������ɣ���ƵIJ���ʵ�鷽����ͼ��ʾ��
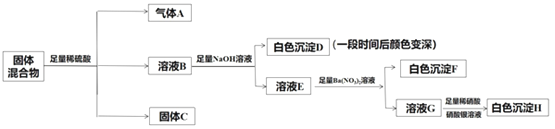
����˵����ȷ����
A.����A���ٺ���SO2��H2�е�һ��
B.����C���ܺ���BaSO4����Ag2SO4
C.�ù���������Fe2O3��Fe����������һ��
D.�ù�������һ������BaCl2���������ʶ���ȷ��
���𰸡�C
��������
��ʵ�鷽����������ҺG�м��������ữ��AgNO3��Һ�����ɰ�ɫ����������ΪAgCl��ԭ������һ������BaCl2��һ������NaBr����ҺB�м���NaOH��Һ���а�ɫ�������ɣ��˳���һ��ʱ�����ɫ�������ΪFe(OH)2��˵����ҺB�к���Fe2+��ԭ�����п��ܺ���Fe(�����ᷴӦ����Fe2+��H2)�����ܺ���Fe2O3(�����ᷴӦ���ɵ�Fe3+ȫ����SO32-��ԭΪFe2+)�������ܺ���AgNO3(����������������������H+���ɵ�HNO3�ܱ�Fe��SO32-ȫ����ԭ������Һ��ֻ����Fe2+��������Fe3+)��
A. ����A����ΪH2������ΪSO2������ΪH2��NO�Ļ����������ΪSO2��NO�Ļ������������ΪH2��NO��SO2�Ļ������A����
B. ԭ������һ������BaCl2�������C��һ������BaSO4�����ܺ���Ag2SO4��B����
C. �����������֪�����������в����Ǻ���Fe2O3���Ǻ���Fe�����ܲ���Fe2+��C��ȷ��
D. �ù�������һ������BaCl2��һ��������NaBr��D����
��ѡC��

 Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д� ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д�����Ŀ��CoCO3������ѡ�������������װͿ�ϵ����ϡ��Ժ��ܷ���(��Ҫ�ɷ�CoO��Co2O3��������Al2O3��ZnO������)Ϊԭ���Ʊ�CoCO3��һ�ֹ����������£�
�������� | ��ʼ������pH | ������ȫ��pH |
Co2+ | 7.6 | 9.4 |
Al3+ | 3.0 | 5.0 |
Zn2+ | 5.4 | 8.0 |
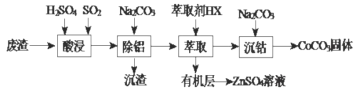
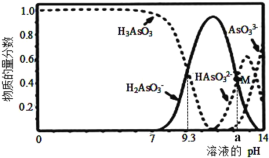
����˵������ȷ���ǣ� ��
A.��������ʱ����������ԭ��Ӧ�����ӷ���ʽCo2O3+SO2+2H+=2Co2++H2O+SO42-
B.����������������Ҫ������Һ pH �ķ�ΧΪ5.0��5.4
C.��ʵ���������ȡ�������õ��IJ���������Ҫ�з�Һ©�����ձ�
D.�ڿ���������CoCO3�������������CO2����ó�����պ��������Ϊ2.41g��CO2�����Ϊ0.672L(��״��)�������������Ļ�ѧʽΪCoO
����Ŀ��H2��һ����Ҫ�������Դ��
��1����֪��CO2(g)+3H2(g)=CH3OH(g)+H2O(g) ��H2=-49.0kJ/mol��
CO(g)+H2O(g)=CO2(g)+H2(g) ��H3=-41.1kJ/mol��H2��ԭCO��Ӧ�ϳɼ״����Ȼ�ѧ����ʽΪ��CO(g)+2H2(g)![]() CH3OH(g) ��H1������H1=___kJ/mol���÷�Ӧ�Է����е�����Ϊ_____��
CH3OH(g) ��H1������H1=___kJ/mol���÷�Ӧ�Է����е�����Ϊ_____��
A.���� B.���� C.�κ��¶�������
��2�����º�ѹ�£����ݻ��ɱ���ܱ������м���1molCO��2.2molH2��������ӦCO(g)+2H2(g)![]() CH3OH(g)��ʵ����ƽ��ʱCO��ת�������¶ȡ�ѹǿ�ı仯��ͼ��ʾ��
CH3OH(g)��ʵ����ƽ��ʱCO��ת�������¶ȡ�ѹǿ�ı仯��ͼ��ʾ��
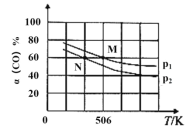
��ѹǿ��p1____p2��(����>��<������=��)
��M��ʱ��H2��ת����Ϊ_____(��������ȷ��0.1%)�� �÷�Ӧ��ƽ�ⳣ��Kp=____(��ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ=��ѹ�����ʵ�������)��
�����и�������Ϊ�жϸ÷�Ӧ�ﵽƽ���־����______(����ĸ)��
A.������ѹǿ���ֲ��� B.2v��(H2)=v��(CH3OH)
C.����������Է����������ֲ��� D.���������ܶȱ��ֲ���
��3��H2��ԭNO�ķ�ӦΪ2NO(g)+2H2(g)![]() N2(g)+2H2O(1)��ʵ���÷�Ӧ���ʵı���ʽΪv=kcm(NO)��cn(H2)(k�����ʳ�����ֻ���¶��й�)
N2(g)+2H2O(1)��ʵ���÷�Ӧ���ʵı���ʽΪv=kcm(NO)��cn(H2)(k�����ʳ�����ֻ���¶��й�)
��ij�¶��£���Ӧ�����뷴Ӧ��Ũ�ȵı仯��ϵ���±���ʾ��
��� | c(NO)/(mol/L) | c(H2)/(mol/L) | v/(mol��L-1��min-1) |
1 | 0.10 | 0.10 | 0.414 |
2 | 0.10 | 0.20 | 1.656 |
3 | 0.50 | 0.10 | 2.070 |
�ɱ������ݿ�֪��m=_____��n=_____��
��������Ӧ���������У�i��2NO(g)+H2(g)=N2(g)+H2O2(1)(����Ӧ)��ii
A.H2O2�Ǹ÷�Ӧ�Ĵ��� B.��Ӧi�Ļ�ܽϸ�
C.�ܷ�Ӧ�����ɷ�Ӧii�����ʾ��� D.��Ӧi��NO��H2����ײ��������Ч
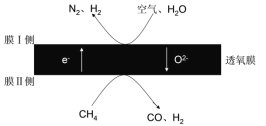
��4��2018���ҹ�ij�����Ŷ�������Ĥ��һ������úϳɰ�ԭ�Ϻͺϳ�Һ̬ȼ�ϵ�ԭ�ϡ��乤��ԭ����ͼ��ʾ��������N2��O2�����ʵ���֮�Ȱ�4��1�ƣ������������У�ĤI������![]() =3����ĤI��ĵ缫����ʽΪ________��
=3����ĤI��ĵ缫����ʽΪ________��