��Ŀ����
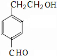
��1��A�ķ���ʽ��
��2����A����NaHCO3��Һ��Ӧ��A�Ľṹ��ʽΪ
��3��д����������Ҫ���A��ͬ���칹��Ľṹ��ʽ��ֻд�����ּ��ɣ�
����A��ȡ������Ŀ��ͬ���ڿ��Ժ�Ũ��ˮ����ȡ����Ӧ��1mol��ͬ���칹�����2mol Br2����ȡ����Ӧ���������������ʣ�
��4����A���Է�����ͼ����ʾת����ϵ��D�ķ���ʽΪC10H12O3��A��C��Ӧ�Ļ�ѧ����ʽ
��5���ס�����װ�þ�������ʵ������C��ȡB��װ�ã���ͼ���ø���ԡ���ȣ����ͷе�290��C���۵�18.17��C�����������¶ȴﵽ��Ӧ�¶�ʱ����ʢ��C��Ũ������Һ����ƿ��������У��ܿ�ﵽ��Ӧ�¶ȣ��ס�����װ�ñȽϣ���װ������Щ�ŵ�
��6������A�ͱ��ӵĻ������ǵ����ʵ���֮��Ϊ n mol���û������ȫȼ������a L CO2��b g H2O���������Ϊ��״���µ����������������A�����ʵ���Ϊx mol���г�x �ļ���ʽ
| 152��31.58% |
| 16 |
| 152��(1-31.58%) |
| 13 |
��2��A��FeCl3��Һ����ɫ��˵�������к��з��ǻ���A����NaHCO3��Һ��Ӧ��˵�������к����Ȼ��������ϵ�һ��ȡ���������֣�˵���ṹ�Գƣ�
��3������A��ȡ������Ŀ��ͬ��˵����������ȡ�������ڿ��Ժ�Ũ��ˮ����ȡ����Ӧ˵�����з��ǻ���1mol��ͬ���칹�����2mol Br2����ȡ����Ӧ��˵�����ǻ�����һ�������Ŵ��ڶ�Ϊ����λ���������������ʣ�˵�������������ݴ��ж���ͬ���칹��ṹ��
��4����A+C��D+H2O��ȷ��C�ķ���ʽ�����ȷ��C��A�ķ�Ӧ��
��5����װ�������ȶ���
��6������̼ԭ�ӻ���ԭ���غ����xֵ��
| 152��31.58% |
| 16 |
| 152��(1-31.58%) |
| 13 |
��2��A����ʽΪC8H8O3��A��FeCl3��Һ����ɫ��˵�������к��з��ǻ���A����NaHCO3��Һ��Ӧ��˵�������к����Ȼ��������ϵ�һ��ȡ���������֣�˵���ṹ�Գƣ���ṹ��ʽӦΪ
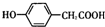 ��A��NaHCO3��Һ��Ӧ�Ļ�ѧ����ʽΪ
��A��NaHCO3��Һ��Ӧ�Ļ�ѧ����ʽΪ ��
���ʴ�Ϊ��
 ��
��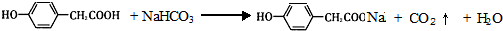 ��
����3������A��ȡ������Ŀ��ͬ��˵����������ȡ�������ڿ��Ժ�Ũ��ˮ����ȡ����Ӧ˵�����з��ǻ���1mol��ͬ���칹�����2mol Br2����ȡ����Ӧ��˵�����ǻ�����һ�������Ŵ��ڶ�Ϊ����λ���������������ʣ�˵�����������������������A��ͬ���칹��Ϊ��
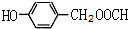 ��
��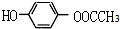 ��
�� ��
��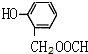 ��
��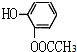 ��
��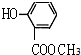 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��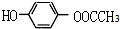 ��
�� ��
�� ��
�� ��
�� ��
����4����A+C��D+H2O��֪��C8H8O3+C��C10H12O3+H2O����C�ķ���ʽΪC2H6O����ΪCH3CH2OH����Ӧ�ķ���ʽΪ
 ��
���ʴ�Ϊ��
 ��
����5��CΪ�Ҵ�����BΪ��ϩ��Ӧ��Ũ���������·�����ȥ��Ӧ���ɣ��Աȼ��ң�������ԡ���ȵķ����������ڿ����¶ȣ������ȶ������ٸ���Ӧ�ķ�����
�ʴ�Ϊ�������ڿ����¶ȣ������ȶ������ٸ���Ӧ�ķ�����
��6��A�ķ���ʽΪC8H8O3�����ӵķ���ʽΪC6H6O��
��A�����ʵ���Ϊx��A�ͱ�����ȫȼ�ն����ɶ�����̼��n��CO2��=n��C��=
| aL |
| 22.4L/mol |
8x+6��n-x���T
| a |
| 22.4 |
| a |
| 44.8 |
��n��H��=��
| b |
| 18 |
| b |
| 9 |
| b |
| 9 |
| b |
| 18 |
�ʴ�Ϊ��x=
| a |
| 44.8 |
| b |
| 18 |

��17�֣��л���A��̼���⡢������Ԫ����ɣ����������Ƿ��͵õ���Ҳ�ɴ���ţ������ȡ��������AΪ��ɫճ��Һ�壬������ˮ��Ϊ�о�A�������ṹ������������ʵ�飺
|
��1����ȡA 9.0g������ʹ�������������ܶ�����ͬ������H2��45���� |
��1���л���A����Է�������Ϊ
|
|
��2������9.0gA��������O2���ȼ�գ���ʹ���������ͨ����ʯ�ҡ���ˮ����ͭ��ĩ������ʯ��ˮ�����ּ�ʯ������14.2g������ͭ��ĩû�б�����ʯ��ˮ����10.0g��ɫ�������ɣ������صļ�ʯ���м��������������4.48L��ɫ��ζ���壨��״������ |
��2��9.0g�л���A��ȫȼ��ʱ�������㣺 ����CO2��Ϊ mol�� ���ɵ�H2O g�� �л���A�ķ���ʽ �� |
|
��3����������ײⶨ��֤ʵ���к���-OH����-COOH���ţ�C-H������˴Ź�������������壬�����Ϊ1�s3�s1�s1�� |
��3��A�Ľṹ��ʽ
|
|
��4����������ײⶨ��A��һ��ͬ���칹���У�����-OH����������ȩ����C-O������˴Ź�������������壬�����Ϊ1�s2�s1�s1�s1�� |
��4��A��ͬ���칹��Ľṹ��ʽ
|
|
��5�������������ײⶨ��A��һ��ͬ���칹���У�����-OH����������C=O��C-O���� ��˴Ź�������������壬�����Ϊ1�s2�� |
��5��A��ͬ���칹��Ľṹ��ʽ
|
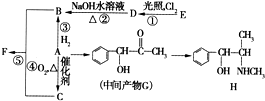 �л���A����Ҫ�Ļ����ϳ�ԭ�ϣ���ҽҩ��Ⱦ�Ϻ����ϵ���ҵ���Ź㷺��Ӧ�ã���A�Ƶ�ijҩ��H��ת����ϵ��ͼ��ʾ��A��G��G��H�ķ�Ӧ�����Ͳ��ַ�Ӧ������ȥ����
�л���A����Ҫ�Ļ����ϳ�ԭ�ϣ���ҽҩ��Ⱦ�Ϻ����ϵ���ҵ���Ź㷺��Ӧ�ã���A�Ƶ�ijҩ��H��ת����ϵ��ͼ��ʾ��A��G��G��H�ķ�Ӧ�����Ͳ��ַ�Ӧ������ȥ����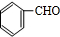
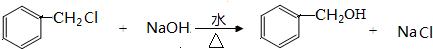
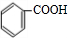 +
+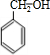
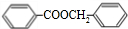 +H2O
+H2O