��Ŀ����
����Ŀ������������������ڹ�ũҵ�������й㷺Ӧ�á�
��1�������⣨MoS2)����Ҫ�Ĺ�������
�����Ϊ2L�ĺ����ܱ������м���0.1molMoS2��0.2molNa2CO3��������0.4molH2��
������Ӧ:MoS2(s)+2Na2CO3(s)+4H2(g)![]() Mo(s) +2CO(g) + 4H2O(g) + 2Na2S(s) ��H =akJ mol-1������ڲ�ͬ�¶��´ﵽƽ��ʱ����������ʵ���������ͼ��ʾ��
Mo(s) +2CO(g) + 4H2O(g) + 2Na2S(s) ��H =akJ mol-1������ڲ�ͬ�¶��´ﵽƽ��ʱ����������ʵ���������ͼ��ʾ��

��a________0���<����>����=��,��ͬ����
�������ڵ���ѹ��P��________Q�㡣
��P���Ӧ�¶��£�H2��ƽ��ת����Ϊ________��ƽ�ⳣ��K=________��
��2����ͭ����Ҫ�ɷ���Cu2S)��ұ�������л����������SO2����֪ұ�������в��ַ�ӦΪ��
��2Cu2S(s)+3O2(g)=2Cu2O(s)+2SO2(g) ��H=-768.2kJ/mol
��2Cu2O+Cu2S(s)=6Cu(s)+SO2(g) ��H=+116kJ/mol����Cu2S��O2��Ӧ����Cu��SO2���Ȼ�ѧ����ʽΪ___________________________��
��3�����մ���SO2�ķ���֮һ���ð�ˮ����ת��ΪNH4HSO3����֪������ Kb(NH3H2O) =1.5��l0-5 Ka1(H2SO3) =1.6��l0-2 Ka2(H2SO3)=1��10-7�������չ����а�ˮ��SO2ǡ����ȫ��Ӧ����������Һ�ڳ����µ�pH________7(�>���� <����=������ͬ������Һ��c(SO32-)________c(H2SO3)��

��4����500��������立ֽ��õ�4�ֲ���京�����ʵ����ʵ�����ʱ��ı仯����ͼ��ʾ���������������立ֽ�Ļ�ѧ����ʽΪ_________________________��
���𰸡� > < 50% 0.0025 Cu2S(s)+O2(g)=2Cu(s)+SO2(g) ��H= -217.4kJ/mol < > 3(NH4)2SO4![]() N2��+4NH3��+3SO2��+6H2O��
N2��+4NH3��+3SO2��+6H2O��
��������(1)������ͼ��P����Q�㣬����������Q���൱�������¶ȣ����������������С��������ӦΪ���ȷ�Ӧ����a��0���ʴ�Ϊ������
��Q���P��������ı���������Ӧ���ʴ���P�㣬�ʲ���������࣬ѹǿ�������ڵ���ѹ��P�㣼Q�㣬�ʴ�Ϊ������
���������������㣬��MoS2�仯�����ʵ���Ϊx��
MoS2(s)+2Na2CO3(s)+4H2(g)Mo(s)+2CO(g)+4H2O(g)+2Na2S(s)��
��ʼ��mol��0.1 0.2 0.4 0 0 0 0
��Ӧ x 2x 4x x 2x 4x 2x
ƽ�� 0.1-x 0.2-2x 0.4-4x x 2x 4x 2x
P��ʱ���������ʵ�������Ϊ40%����![]() ��100%=40%��x=0.05��H2��ƽ��ת����=
��100%=40%��x=0.05��H2��ƽ��ת����=![]() ��100%=0.05��100%=50%�� P���ƽ�ⳣ��K=
��100%=0.05��100%=50%�� P���ƽ�ⳣ��K=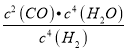 =
=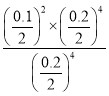 =2.5��10-3���ʴ�Ϊ��50%��0.0025��
=2.5��10-3���ʴ�Ϊ��50%��0.0025��
(2)��2Cu2S(s)+3O2(g)=2Cu2O(s)+2SO2(g) ��H=-768.2kJ/mol ����2Cu2O+Cu2S(s)=6Cu(s)+SO2(g) ��H=+116kJ/mol�����ݸ�˹���ɣ�����+���ã�3Cu2S(s)+3O2(g)=6Cu(s)+3SO2(g) ��H=(-768.2kJ/mol)+(+116kJ/mol)=-652.2 kJ/mol����Cu2S(s)+O2(g)=2Cu(s)+SO2(g) ��H= -217.4kJ/mol���ʴ�Ϊ��Cu2S(s)+O2(g)=2Cu(s)+SO2(g) ��H= -217.4kJ/mol��
(3)���ݳ����� Kb(NH3H2O) =1.5��l0-5 Ka1(H2SO3) =1.6��l0-2 Ka2(H2SO3)=1��10-7����֪笠����ӵ�ˮ��ƽ�ⳣ��=![]() =
=![]() ��l0-9��������������ӵĵ���̶ȴ���笠����ӵ�ˮ��̶ȣ�NH4HSO3��Һ�����ԣ�pH��7����������������ӵĵ���Ϊ������Һ��c(SO32-)��c(H2SO3) ����Ϊ��< ��>��
��l0-9��������������ӵĵ���̶ȴ���笠����ӵ�ˮ��̶ȣ�NH4HSO3��Һ�����ԣ�pH��7����������������ӵĵ���Ϊ������Һ��c(SO32-)��c(H2SO3) ����Ϊ��< ��>��
(4)��500��������立ֽ�����еõ�4�ֲ���京��������ʱ��仯��ϵ��ͼ����ʾ������立ֽ����ɵ��������������������ˮ����ϵ����غ��ԭ���غ���ƽ��д�õ���ѧ����ʽΪ��3(NH4)2SO4![]() N2��+4NH3��+3SO2��+6H2O�����ʴ�Ϊ��3(NH4)2SO4
N2��+4NH3��+3SO2��+6H2O�����ʴ�Ϊ��3(NH4)2SO4![]() N2��+4NH3��+3SO2��+6H2O������
N2��+4NH3��+3SO2��+6H2O������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ�����ᣨH2C2O4)��һ����Ҫ���л�����ԭ�ϡ�Ϊ̽���������ȡ�����ʼ�Ӧ�ã���������ʵ�顣
ʵ����ᾧ����Ʊ�
ʵ������������������ˮ��Һ���Ʊ����ᣬװ�D��ͼ��ʾ��

��һ�����ĵ���ˮ��Һ����������ƿ��
�ڿ��Ʒ�Ӧ�¶�55-60�棬�߽�������μ�һ�����Ļ����
�۷�Ӧ3Сʱ����ȴ�����˺����ؽᾧ�õ����ᾧ�塣
��1����ͼʵ��װ��������������Ϊ________������ˮ�Ľ�����________������a������b�� ����
��2����������Ƿ�ˮ����ȫ����Ҫ���Լ�Ϊ______________��
ʵ���̽�����������Ը��������Һ�ķ�Ӧ
��3���������Һ����μ��������ữ�ĸ��������Һʱ���ɹ۲쵽��Һ���Ϻ�ɫ��Ϊ������ɫ�����Ʋ������ܾ���________�ԡ���Ӧ���ʿ�ʼ�����������ӿ죬���ܵ�ԭ����_________��д����Ӧ�����ӷ���ʽ________________��
ʵ��������ʵ�Ӧ��
���ñ�H2C2O4������KMnO4��Һ��Ӧ̽���������Ի�ѧ��Ӧ���ʵ�Ӱ�졣��ʵ��ʱ���ȷֱ���ȡ������Һ��Ȼ�����Թ���Ѹ����Ͼ��ȣ���ʼ��ʱ��ͨ���ⶨ��ɫ����ʱ�����жϷ�Ӧ�Ŀ�������Ʒ������£�
��� | H2C2O4��Һ | ���Ը��������Һ | �¶�/�� | ||
Ũ��/molL-1 | ���/mL | Ũ��/molL-l | ���/mL | ||
�� | 0.10 | 2.0 | 0.010 | 4.0 | 25 |
�� | 0.20 | 2.0 | 0.010 | 4.0 | 25 |
�� | 0.20 | 2.0 | 0.010 | 4.0 | 50 |
��4��Ϊ�˹۲쵽��ɫ��ȥ��H2C2O4��KMnO4��Һ��ʼ�����ʵ�����Ҫ����Ĺ�ϵΪn(H2C2O4)��n(KMnO4)��________��
��5��̽���¶ȶԻ�ѧ��Ӧ����Ӱ���ʵ������___________��̽����Ӧ��Ũ�ȶԻ�ѧ��Ӧ����Ӱ���ʵ������__________��
ʵ��������ᾧ���нᾧˮ�ⶨ
���ᾧ��Ļ�ѧʽ�ɱ�ʾΪH2C2O4xH2O��Ϊ�ⶨx��ֵ����������ʵ�飺
�ٳ�ȡ6.3gij���ᾧ�����100. 0mL��ˮ��Һ��
��ȡ25.00mL������Һ������ƿ�У���������ϡH2SO4,��Ũ��Ϊ0.5ml/L��KMnO4��Һ�ζ����ζ��յ�ʱ����KMnO4�����Ϊ10.00mL��
��6������x= ________��
