��Ŀ����
����Ŀ�������ϩ����Ҫ���л�����ԭ�ϣ��Ʊ�����ϩ�漰�ķ�Ӧ���£�
��.��۷�Ӧ�� ��H��0
��H��0
��.�⻯��Ӧ�� ��H=-100.5kJ��mol-1
��H=-100.5kJ��mol-1
��.����Ӧ��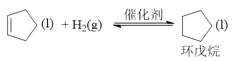 ��H=-109.4kJ��mol-1
��H=-109.4kJ��mol-1
��1����Ӧ![]() ��H=__kJ��mol-1��
��H=__kJ��mol-1��
��2����۷�Ӧ���ڸ��������н��С�
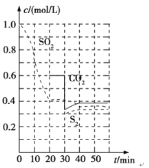
��������������ʱ�����������˫�����ϩƽ��ת���ʵ�������__��__��
��ʵ�������г�ͨ��ˮ�����Խ���˫�����ϩ�ķе㡣ij�¶��£�ͨ����ѹΪ70kPa��˫�����ϩ��ˮ�������ﵽƽ�����ѹΪ115kPa��˫�����ϩ��ת����Ϊ90%������ϵ��PH2O=__kPa��
��3���о������ϩ�⻯�Ʊ�����ϩ�ڲ�ͬ�¶��´����Ļ��ԡ��±�����Pd/Al2O3Ϊ��������ͬ��Ӧʱ���ò�ͬ�¶ȵ����ݣ�����������ͬ����
�¶�/�� | 25 | 30 | 35 | 40 | 45 |
�����ϩת����/% | 84.9 | 93.9 | 100.0 | 100.0 | 100.0 |
����ϩѡ����/% | 81.3 | 88.6 | 75.5 | 60.6 | 51.3 |
˵����ѡ����=![]() ��100%������=
��100%������=![]() ��100%��
��100%��
�������������������Ļ��ԡ�
����ѵķ�Ӧ�¶�Ϊ__��ѡ����¶ȵ�ԭ����__��
�ڱ���ʵ�����ݱ����������¶ȣ������ϩת������߶�����ϩѡ���Խ��ͣ���ԭ����__��
���𰸡�-209.9 �����¶� ��Сѹǿ 20 30�� ���¶��£�����������Ļ�����û���¶���ת���ʽϸߣ�ѡ���Ժ� �¶����ߣ��������ķ�Ӧ���ʾ��ӿ죬���Ԣ��ķ�Ӧ���ʵ�Ӱ��С�ڢ�
��������
(1)��֪����.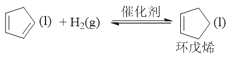 ��H=-100.5kJ��mol-1����.
��H=-100.5kJ��mol-1����.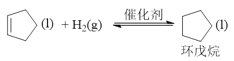 ��H=-109.4kJ��mol-1���ɸ�˹���ɿ�֪����+����
��H=-109.4kJ��mol-1���ɸ�˹���ɿ�֪����+����![]() ����ɼ���H��
����ɼ���H��
(2)�ٽ��Ӱ������ط������ı������ٽ�ƽ�������ƶ����ﵽ���˫�����ϩƽ��ת���ʵ�Ŀ�ģ�
���跴Ӧǰ˫�����ϩ��ˮ�����������ʵ���Ϊn��ѹǿΪ�������ʵ��������ȣ���Ӧǰ��ѹǿ70kPa��˫�����ϩ��ˮ�������ﵽƽ�����ѹΪ115kPa����ƽ��ʱ�����������ʵ���Ϊ��![]() mol=1.64nmol�����ʵ���������0.64nmol�������
mol=1.64nmol�����ʵ���������0.64nmol�������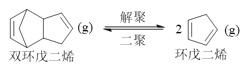 ��֪�μӷ�Ӧ��˫�����ϩΪ0.64nmol����˫�����ϩ��ת����Ϊ90%��֪ԭ���������˫�����ϩΪ
��֪�μӷ�Ӧ��˫�����ϩΪ0.64nmol����˫�����ϩ��ת����Ϊ90%��֪ԭ���������˫�����ϩΪ![]() =0.714nmol���ݴ˼���PH2O��
=0.714nmol���ݴ˼���PH2O��
(3)���ۺϱ������ݷ��������ϩת���ʺͻ���ϩѡ����������ʱ����Ӧ���¶ȣ�
�ڸ����¶ȶԷ�Ӧ������������Ӱ���жϡ�
(1)��֪����. ��H=-100.5kJ��mol-1����.
��H=-100.5kJ��mol-1����. ��H=-109.4kJ��mol-1���ɸ�˹���ɿ�֪����+����
��H=-109.4kJ��mol-1���ɸ�˹���ɿ�֪����+����![]() ����ʱH=(-100.5kJ��mol-1)+(-109.4kJ��mol-1)=-209.9 kJ��mol-1��
����ʱH=(-100.5kJ��mol-1)+(-109.4kJ��mol-1)=-209.9 kJ��mol-1��
(2)���� ��H��0��֪�����������¶Ȼ��Сѹǿ�ȴ�ʩ���ɴٽ�ƽ�������ƶ����ﵽ���˫�����ϩƽ��ת���ʵ�Ŀ�ģ�
��H��0��֪�����������¶Ȼ��Сѹǿ�ȴ�ʩ���ɴٽ�ƽ�������ƶ����ﵽ���˫�����ϩƽ��ת���ʵ�Ŀ�ģ�
���跴Ӧǰ˫�����ϩ��ˮ�����������ʵ���Ϊn��ѹǿΪ�������ʵ��������ȣ���Ӧǰ��ѹǿ70kPa��˫�����ϩ��ˮ�������ﵽƽ�����ѹΪ115kPa����ƽ��ʱ�����������ʵ���Ϊ��![]() mol=1.64nmol�����ʵ���������0.64nmol�������
mol=1.64nmol�����ʵ���������0.64nmol������� ��֪�μӷ�Ӧ��˫�����ϩΪ0.64nmol����˫�����ϩ��ת����Ϊ90%��֪ԭ���������˫�����ϩΪ
��֪�μӷ�Ӧ��˫�����ϩΪ0.64nmol����˫�����ϩ��ת����Ϊ90%��֪ԭ���������˫�����ϩΪ![]() =0.714nmol����ˮ���������ʵ���Ϊnmol-0.714nmol=0.286nmol����ʱPH2O=
=0.714nmol����ˮ���������ʵ���Ϊnmol-0.714nmol=0.286nmol����ʱPH2O=![]() ��115KPa��20KPa��
��115KPa��20KPa��
(3)���ɱ������ݿ�֪����30��ʱ������������Ļ�����ã��Ҵ�ʱ�����ϩת���ʽϸߣ�����ϩѡ������ã�����ѵķ�Ӧ�¶�Ϊ30�棻
���¶����ߣ��������ķ�Ӧ���ʾ��ӿ죬�������ݱ��������¶ȣ������ϩת������߶�����ϩѡ���Խ��ͣ������ԭ�����¶ȶԶԢ��ķ�Ӧ���ʵ�Ӱ��С�ڢ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��A��B��M��N�����л�����й���Ϣ���±���ʾ��
A | B | M | N |
����ģ��Ϊ�� | ���ģ��Ϊ�� | ����ʽ�ṹΪ�� | �������Ʒ�Ӧ����������NaOH��Һ��Ӧ��������M��Ӧ������Է�������Ϊ100���� |
��ش�
��1��A��һ�������¿ɺϳɰ�װ���ϣ���Ӧ�Ļ�ѧ����ʽΪ____________��
��2����Ni�������������£�B����������ʱ��Ӧ�Ļ�ѧ����ʽΪ______________��
��3��M�ķ���ʽΪ__________��M��N��Ӧ�Ļ�ѧ����ʽΪ_______________��
��4����������������M��ͬ���칹��Ľṹ��ʽΪ______________��
I����״ ii��ֻ��һ�ֹ����� iii����������������ͭ�ڼ�������������ש��ɫ����