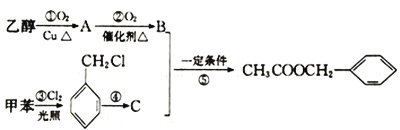
��Ŀ����
����Ŀ��A��B��M��N�����л�����й���Ϣ���±���ʾ��
A | B | M | N |
����ģ��Ϊ�� | ���ģ��Ϊ�� | ����ʽ�ṹΪ�� | �������Ʒ�Ӧ����������NaOH��Һ��Ӧ��������M��Ӧ������Է�������Ϊ100���� |
��ش�
��1��A��һ�������¿ɺϳɰ�װ���ϣ���Ӧ�Ļ�ѧ����ʽΪ____________��
��2����Ni�������������£�B����������ʱ��Ӧ�Ļ�ѧ����ʽΪ______________��
��3��M�ķ���ʽΪ__________��M��N��Ӧ�Ļ�ѧ����ʽΪ_______________��
��4����������������M��ͬ���칹��Ľṹ��ʽΪ______________��
I����״ ii��ֻ��һ�ֹ����� iii����������������ͭ�ڼ�������������ש��ɫ����
���𰸡� ![]()
![]() C3H4O2 CH2=CHCOOH + CH3CH2OH
C3H4O2 CH2=CHCOOH + CH3CH2OH![]() CH2=CHCOOCH2CH3 + H2O
CH2=CHCOOCH2CH3 + H2O ![]()
����������1��ͨ������ģ���ж�AΪ��ϩ����ϩ�ϳɾ���ϩ�ķ���ʽΪ��![]() ��
��
��2��ͨ��B�����ģ���ж�BΪ���������������ȷ����ӳɷ�Ӧ�ķ���ʽΪ��![]() ��
��
��3��ͨ��M�ļ���ʽ�ж�MΪ��ϩ�ᣬ����ʽΪ��C3H4O2��N�����Ʒ�Ӧ����������NaOH��Һ��Ӧ˵�����ǻ�������M��Ӧ������Է�������Ϊ100����ΪCH2=CHCOOCH2CH3������NΪCH3CH2OH��M��N��Ӧ�Ļ�ѧ����ʽΪCH2=CHCOOH + CH3CH2OH![]() CH2=CHCOOCH2CH3 + H2O��
CH2=CHCOOCH2CH3 + H2O��
��4����ֻ��һ�ֹ����ź���������������ͭ�ڼ�������������ש��ɫ�����жϸ����ʺ�������ȩ����ͬʱ����״���жϸ����ʵĽṹ��ʽΪ��![]() ��
��