��Ŀ����
����Ŀ��������(Co)���ڵ������ڵ�VIII��Ԫ�أ��������ܻ�������������;���ش��������⣺
(1)��̬Coԭ�Ӽ۲���ӵĵ����Ų�ͼ(�������ʽ)Ϊ________________��
(2)Co�������[Co(NH3)5Cl]SO4��һ����Ҫ�Ļ����
��SO42-�Ŀռ����幹��Ϊ___________������Sԭ�ӵ��ӻ����������___________��д��һ����SO42-��Ϊ�ȵ�����ķ��ӵĻ�ѧʽ:______________��
��[Co(NH3)5Cl]SO4�У�N��O��S����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ_______________��
��[Co(NH3)5Cl]SO4�У�����___________(����ĸ)
a.���Ӽ� b.������ c.��λ�� d.�Ǽ��Լ� e.���Լ�
(3)���軯��{K3 [Fe(CN)6]}��Һ�Ǽ���Fe2+���õ��Լ���
��1mol [Fe(CN)6]3-��![]() ������ĿΪ__________��
������ĿΪ__________��
��CN-������������γ�����̼ԭ���ṩ�µ��Ӷԣ������ǵ�ԭ�ӣ���ԭ����____________��
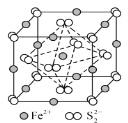
��FeS2����ľ�����ͼ��ʾ�������߳�Ϊa nm��FeS2��Է�������ΪM�������ӵ�������ֵΪNA���侧���ܶȵļ������ʽΪ______gcm-3��������Fe2+λ��S22-���γɵ�������������ģ�����������ı߳�Ϊ_________nm��
���𰸡�![]() �������� sp3 CCl4�������������𰸣� N>O>S ace 12NA ̼��ԭ�Ӱ뾶���ڵ���ԭ�Ӱ뾶������̼�ṩ�µ��ӶԱȵ�ǿ
�������� sp3 CCl4�������������𰸣� N>O>S ace 12NA ̼��ԭ�Ӱ뾶���ڵ���ԭ�Ӱ뾶������̼�ṩ�µ��ӶԱȵ�ǿ ![]()
![]() a
a
��������
(1)Co��27��Ԫ�أ����ڵ������ڵ�VIII�壬�۵����Ų�ʽΪ3d74s2���������ԭ�������ع�����д�۵����Ų�ͼ��
(2)�ٸ���SO42-����ԭ�Ӻ��еĹ��ۼ�������µ��ӶԸ���֮��ȷ����ռ乹�ͺ��ӻ���ʽ��ԭ�Ӹ�������Ҽ۵�������ȵ���Ϊ�ȵ����壻��ͬ��������Ԫ������ԭ����������һ�����ܳ���������ƣ�����A��͵���A��Ԫ�ط�����ͬ����Ԫ�أ�����ԭ����������һ����������[Co(NH3)4]SO4�У��������뵪ԭ��֮���γ���λ��������������Nԭ����Hԭ��֮���γɼ��Լ��������֮��Ϊ���Ӽ���
(3)��[Fe(CN)6]3-�к���6����λ����6��C��N�����ڸ���ԭ�Ӱ뾶������𣻢۸��ݾ����߳����㾧�����=(a��10-7cm)3�����ݾ�̯�����㾧���к��е�Fe2+������S22-�������ٸ��ݾ����ܶ�=![]() ���㣻������Fe2+λ��S22-���γɵİ���������ģ�����������ı߳����������ζԽ��ߵij��ȼ��㡣
���㣻������Fe2+λ��S22-���γɵİ���������ģ�����������ı߳����������ζԽ��ߵij��ȼ��㡣
(1)Co��27��Ԫ�أ����ڵ������ڵ����壬�۵����Ų�ʽΪ3d74s2���������ԭ�������ع��۵����Ų�ͼΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(2)��SO42-��Sԭ�Ӽ۲���Ӷ�=4+![]() (6+2-4��2)=4���Ҳ����µ��Ӷԣ����������幹�����������壬��ԭ�Ӳ�ȡsp3�ӻ���ԭ�Ӹ�������Ҽ۵�������ȵ���Ϊ�ȵ����壬��SO42-��Ϊ�ȵ��������Ϊ��CCl4��PO43-��SiO44-�ȣ��ʴ�Ϊ���������壻sp3��CCl4(��PO43-��SiO44-)��
(6+2-4��2)=4���Ҳ����µ��Ӷԣ����������幹�����������壬��ԭ�Ӳ�ȡsp3�ӻ���ԭ�Ӹ�������Ҽ۵�������ȵ���Ϊ�ȵ����壬��SO42-��Ϊ�ȵ��������Ϊ��CCl4��PO43-��SiO44-�ȣ��ʴ�Ϊ���������壻sp3��CCl4(��PO43-��SiO44-)��
��ͬ��������Ԫ������ԭ����������һ�����ܳ���������ƣ�����A��͵���A��Ԫ�ط�����ͬ����Ԫ�أ�����ԭ����������һ�����ܼ�С������N��O��S ����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ��N��O��S���ʴ�Ϊ��N��O��S��
��[Co(NH3)5Cl]SO4�У��������뵪ԭ��֮���γ���λ��������������Nԭ����Hԭ��֮���γɼ��Լ��������֮��Ϊ���Ӽ����������к��еĻ�ѧ���������У����Ӽ�����λ�������Լ����ʴ�Ϊ��ace��
(3)��[Fe(CN)6]3-�к���6����λ����6��C��N�������1mol [Fe(CN)6]3-��![]() ��12mol����ĿΪ12NA���ʴ�Ϊ��12NA��
��12mol����ĿΪ12NA���ʴ�Ϊ��12NA��
��CN-������������γ�����̼ԭ���ṩ�µ��Ӷԣ������ǵ�ԭ�ӣ�ԭ����̼��ԭ�Ӱ뾶���ڵ���ԭ�Ӱ뾶������̼�ṩ�µ��ӶԱȵ�ǿ���ʴ�Ϊ��̼��ԭ�Ӱ뾶���ڵ���ԭ�Ӱ뾶������̼�ṩ�µ��ӶԱȵ�ǿ��
�۾����߳�Ϊanm=a��10-7cm���������=(a��10-7cm)3���þ�����Fe2+����=1+12��![]() =4��S22-����=8��
=4��S22-����=8��![]() +6��
+6��![]() =4���侧���ܶ�=
=4���侧���ܶ�=![]() =
=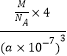 g/cm3=
g/cm3=![]() g/cm3��������Fe2+λ��S22-���γɵİ���������ģ������������ƽ��Խ���Ϊ
g/cm3��������Fe2+λ��S22-���γɵİ���������ģ������������ƽ��Խ���Ϊ![]() anm������������ı߳�Ϊ
anm������������ı߳�Ϊ![]() anm���ʴ�Ϊ��
anm���ʴ�Ϊ��![]() ��
��![]() a��
a��

����Ŀ���±���Ԫ�����ڱ���һ���֣���Ա��е���������Ԫ�أ���Ԫ�ط��Ż�ѧʽ��ջش��������⣺
���� | IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
�� | �� | �� | ||||||
�� | �� | �� | �� | �� | �� | �� | ||
�� | �� | �� |
��1����������Ԫ���У�ԭ�Ӱ뾶������ �������ӵ�ԭ�ӽṹʾ��ͼΪ
��2��Ԫ�ص�����������Ӧ��ˮ������������ǿ�� ��������ǿ���� �������Ե����������� ��
��3����Ҫ��д�������������ʵĵ���ʽ�������⻯�� ���������������Ķ�Ӧ��ˮ���� ��
��4�����������ĵ����У���ѧ���ʽϻ��õ��� ������ʲô��ѧ��Ӧ˵������ʵ��д����Ӧ�Ļ�ѧ����ʽ���� ��