��Ŀ����
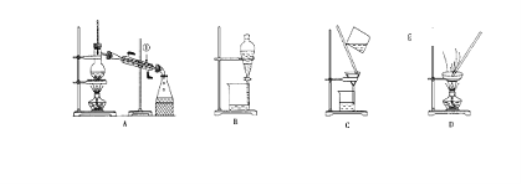
����Ŀ����п��ZnS����һ����Ҫ�Ļ���ԭ�ϣ�������ˮ��������п�ķ���п����ȡ�乤��������ͼ��ʾ��

��1��Ϊ���п�ҵĽ�ȡ�ʣ��ɲ��õķ�����______________������ţ���
����ĥ �ڶ�ν�ȡ �������¶� �ܼ�ѹ �ݽ���
��2����������������е������ǣ�д��ѧʽ��_____________��
��3��������пɵ�Cd���ʣ�Ϊ���������µ����ʣ��Լ�XӦΪ________��
��4������������Ի���Na2SO4����ȡNa2S��
�ټ���ZnS�����Ƿ�ϴ�Ӹɾ��ķ�����__________________��
��Na2S���ɵ����ʵ�����Na2SO4��CH4�ڸ��¡�������������ȡ����ѧ��Ӧ����ʽΪ_______��
����֪Na2SO4��10H2O��Na2SO4���ܽ�����¶ȱ仯������ͼ������Һ�еõ�Na2SO4��10H2O�IJ���������_________________________________��

��5�������������ZnCO3Ϊbmol�����������CdΪdmol�����õ�VL�����ʵ���Ũ��Ϊcmol/L��Na2SO4��Һ��������������п���к���пԪ�ص�����Ϊ__________��
���𰸡� �٢ڢۢ� Fe(OH)3 Zn ȡ�������һ��ϴ��Һ���Թ��У��ȼ���HCl�ữ���ټ����Ȼ��������ް�ɫ�������ɣ�����ϴ�� Na2SO4+CH4![]() Na2S+CO2+2H2O ����Ũ������ȴ�ᾧ�����ˣ�ϴ�ӣ����� 65(Vc-b-d)
Na2S+CO2+2H2O ����Ũ������ȴ�ᾧ�����ˣ�ϴ�ӣ����� 65(Vc-b-d)
�������������������������п�ķ���п���Ʊ�ZnS�Ĺ�������Ϊ���壬�������̵ķ���������ʵ���������ѧ���㡣
��1������I��п���м���ϡ����õ���ȡҺ����ȡҺ�к�Zn2+��Cd2+��Fe3+��Fe2+�����٣���ĥ������п��������Һ�ĽӴ��������߽�ȡ�ʣ��ڣ���ν�ȡ��߽�ȡ�ʣ��ۣ������¶ȼӿ��ȡ���ʣ���߽�ȡ�ʣ��ܣ���ȡ����Ϊ�����Һ��ķ�Ӧ��û��������룬��ѹ������߽�ȡ�ʣ��������������п��������Һ�ĽӴ��������߽�ȡ�ʣ���߽�ȡ�ʿɲ��õķ������٢ڢۢݣ���ѡ�٢ڢۢݡ�
��2������II��п�ҽ�ȡҺ�м���H2O2��H2O2��Fe2+������Fe3+��Ȼ���������ZnCO3������Һ��pH��ʹFe3+ת����Fe��OH��3ͨ�����˵ķ�����ȥ������II���������е����ʵĻ�ѧʽΪFe��OH��3��
��3����ҺI�к�Zn2+��Cd2+������III�пɵõ�Cd���ʣ�Ϊ�������������ʣ��Լ�XӦΪZn�������ķ�ӦΪZn+Cd2+=Zn2++Cd��
��4������IVΪ��ZnSO4��Һ�м���Na2S����ZnS��Na2SO4����Ӧ�Ļ�ѧ����ʽΪNa2S+ZnSO4=ZnS��+Na2SO4��
������ZnS�����Ƿ�ϴ�Ӹɾ�����������ϴ��Һ���Ƿ�SO42-���ɣ�ʵ�鷽���ǣ�ȡ�������һ��ϴ��Һ���Թ��У��ȼ���HCl�ữ���ټ���BaCl2�����ް�ɫ�������ɣ�����ϴ����
�������ʵ�����Na2SO4��CH4�ڸ��¡����������·�Ӧ����Na2S������ԭ���غ㻹����CO2��H2O����Ӧ�Ļ�ѧ����ʽΪNa2SO4+CH4![]() Na2S+CO2+2H2O��
Na2S+CO2+2H2O��
�������ܽ�����ߣ�����Һ�еõ�Na2SO4��10H2O�IJ��������ǣ�����Ũ������ȴ�ᾧ�����ˣ�ϴ�ӣ����
��5������II�м���bmolZnCO3��ȥFe3+����Һ������bmolZn2+������III��Zn��Cd2+�����û���Ӧ��Zn+Cd2+=Zn2++Cd���õ�dmolCd��ͬʱ��Һ������dmolZn2+������IV�еķ�ӦΪNa2S+ZnSO4=ZnS��+Na2SO4����ҺII��n��Zn2+����=n��Na2SO4��=cVmol������Zn�غ���������п���к���пԪ�ص����ʵ���Ϊ��cV-b-d��mol��п���к���пԪ�ص�����Ϊ65��cV-b-d��g��

 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�