��Ŀ����
����Ŀ����.��ͼ����ѧ��ѧ�г����ڻ����ķ�����ᴿ��װ�ã������װ�ûش����⣺
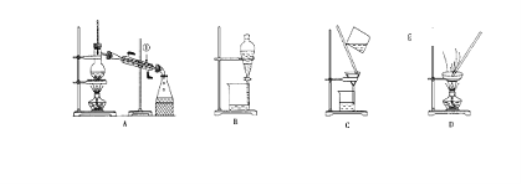
��1�����Ȼ�����Һ�еõ��Ȼ��ع��壬ѡ��װ��____________(�����װ��ͼ����ĸ����ͬ)����ȥ����ˮ�е�Cl�������ʣ�ѡ��װ��____________��
��2���ӵ�ˮ�з����I2��ѡ��װ��_________���÷��뷽��������Ϊ___________��
��3��װ��A�Тٵ�ˮ�Ľ���������_________��A�����в������������Ʒֱ���__________��
��.���ڻ����ķ�����ᴿ�������õķ����У�A����Һ B������ C����ȡ D������ E���ᾧ F�����ȷֽ⣬���и������ʵķ�����ᴿ��Ӧѡ��������������һ�֣�������ĸ��ţ�
��4����ȥCa(OH)2��Һ��������CaCO3__________��
��5������ֲ���ͺ�ˮ__________��
��6����ȥNaCl������������KNO3___________��
��7����ȥCaO������CaCO3__________��
��8����ʳ�þƾ������в�ҩ��ȡ���е���Ч�ɷ�_________��
��9�����յ��CCl4��Һ�е�CCl4__________��
���𰸡�DAB��ȡ�½��ϳ��ƾ��ơ�������ƿ���¶ȼơ������ܡ�ţ�ǹܡ���ƿBAEFCD
��������
����װ��ͼ��֪A������װ�ã�B�Ƿ�Һװ�ã�C�ǹ���װ�ã�D������װ�ã�������ʵ����ʷ������
��1�������ܽ�������ˮ�Ĺ����ˮ�ķ��룬���Ȼ�����Һ�еõ��Ȼ��ع��������������ѡD�����������ʵ��ˮ���ѻӷ������ʺ�ˮ�ķ��룬���ȥ����ˮ�е�Cl�������ʣ���������ѡA��
��2��������ˮ���������ڱ������Ȼ�̼���л��ܼ����ұ������Ȼ�̼��ˮ�������ܣ�������ȡ�����룬��ѡB��
��3��װ��A�Тٵ�������ֱ�������ܣ�ʹ��ʱ�¿ڽ�ˮ�Ͽڳ�ˮ������װ�ù����֪A�в������������Ʒֱ��Ǿƾ��ơ�������ƿ���¶ȼơ������ܡ�ţ�ǹܡ���ƿ��
��4��̼��Ʋ�����ˮ����ѡ����˷����룬��ΪB��
��5��ֲ������ˮ�ֲ㣬��ѡ���Һ�����룬��ΪA��
��6�������ܽ�����¶�Ӱ�첻ͬ����ѡ��ᾧ�����룬��ΪE��
��7��̼��Ƹ��·ֽ�����CaO����ѡ����·ֽⷨ���룬��ΪF��
��8����Ч�ɷ�Ϊ�л�������ھƾ�����÷��뷽��Ϊ��ȡ����ΪC��
��9�������ܣ����е㲻ͬ����ѡ������������Ȼ�̼����ΪD��

����Ŀ����������ƾ���(Na2S2O3��5H2O)�ֳƺ���������ɫ��״��������ˮ���������Ҵ�����������֯��Ư������ȼ������������еĻ�ԭ����
��. Na2S2O3��5H2O���Ʊ�
Na2S2O3��5H2O���Ʊ������ж��֣������������Ʒ��ǹ�ҵ��ʵ�����е���Ҫ������
Na2SO3+S+5H2O![]() Na2S2O3��5H2O
Na2S2O3��5H2O
�Ʊ��������£�
�ٳ�ȡ12.6gNa2SO3��100mL�ձ��У���50mLȥ����ˮ�����ܽ⡣
����ȡ4.0g�����200mL�ձ��У���6mL�Ҵ���ֽ�����Ƚ�����ʪ���ټ���Na2SO3��Һ����ʯ����С�������У����Ͻ�������ۼ���ȫ����Ӧ��
��ֹͣ���ȣ�����Һ����ȴ���2g����̿���������2����(��ɫ)��
�ܳ��ȹ��ˣ�����Һ���������У�_________________��____________________��
�ݹ��ˡ�ϴ�ӣ�����ֽ���ɺ��أ�������ʡ�
��1�������������Ҵ���ʪ��Ŀ����____________________________��
��2������ܳ��ȹ��˵�ԭ��_____________________���ո�Ӧ��ȡ�IJ�����_________________��____________________��
��3�������ϴ�ӹ����У�Ϊ��ֹ�в��ֲ�Ʒ��ʧ��Ӧѡ�õ��Լ�Ϊ__________________________��
��4����Һ�г�Na2S2O3 ��δ��Ӧ��ȫ��Na2SO3�⣬����ܴ��ڵ���������________________�����ɸ����ʵ�ԭ�������____________________________��
��.��Ʒ���ȵIJⶨ
ȷ��ȡ1.00g��Ʒ(��������ƾ����Ħ������Ϊ248g/mol)������������ˮ�ܽ⣬�Ե�����ָʾ������0.1000mol/LI2�ı���Һ�ζ�����Ӧ�����ӷ���ʽΪ��2S2O32-+I2=S4O62-+2I-����¼�������£�
�ζ����� | �ζ�ǰ����(mL) | �ζ������(mL) |
1 | 0.30 | 21.32 |
2 | 0.36 | 22.56 |
3 | 0.10 | 21.08 |
��5���������ò�Ʒ�Ĵ���Ϊ___________(������λ��Ч����)�������ݵĺ������Ϳ�����__________(������ʵ�������������)��
��.��Ʒ��Ӧ��
��6��Na2S2O3 ���������ȼ�������Һ���ױ�Cl2 ����ΪSO42-���÷�Ӧ�����ӷ���ʽΪ _____________________________��



