��Ŀ����
����Ŀ����������п��һ�ֶ���Ե����������ϣ�ijС���Դ�����п��������ͭ�������Ϊԭ��ģ�ҵ������������п���������£�

��֪����������������pH��Χ���±���ʾ��
Zn��OH��2 | Fe��OH��2 | Fe��OH��3 | Cu��OH��2 | |
��ʼ����pH | 5.4 | 7.0 | 2.3 | 4.7 |
��ȫ����pH | 8.0 | 9.0 | 4.1 | 6.7 |
���������գ�
��1��Ϊ�˼ӿ��ȡ�ķ�Ӧ���ʣ����Բ�ȡ�Ĵ�ʩ_________________________________
��2������II�м���H2O2��Һ��������_________________________�������ӷ���ʽ��ʾ����
��3����ZnO����pH���Գ�ȥ�������ʣ�����pH�����˷�Χ��_______________________��
��4������III�м���Zn�۵������ǣ���__________________������һ��������ҺpH��
��5����ʽ̼��п[Zn2(OH)2 CO3]���յĻ�ѧ����ʽΪ________________________________��
��6�������·����ⶨ���û�������п�Ĵ��ȣ��������ʲ����뷴Ӧ����
�� ȡ1.000g��������п����15.00mL 1.000mol��L-1 ������Һ��ȫ�ܽ⣬���뼸�μ��ȡ��� ��Ũ��Ϊ0.5000mol��L-1 �ı�����������Һ�ζ�ʣ�����ᣬ�����յ�ʱ��������������Һ12.00mL���жϵζ��յ�ķ�����___________________________________________�����û�������п�Ĵ���Ϊ________________________
���𰸡����Ȼ��ʵ����������Ũ�Ȼ������2Fe2++H2O2+2H+=2Fe3++2H2O4.1��4.7��ȥ��Һ�е�Cu2+Zn2(OH)2CO3![]() 2ZnO+CO2��+H2O����Һ�ɳ�ɫ��Ϊ��ɫ�Ұ�����ڲ���ɫ��Ϊ�ζ��յ�97.2%
2ZnO+CO2��+H2O����Һ�ɳ�ɫ��Ϊ��ɫ�Ұ�����ڲ���ɫ��Ϊ�ζ��յ�97.2%
��������
������п��������ͭ�����������ϡ�����ܽ⣬���ˣ��õ���Һ�к�����������������ͭ������п������H2O2��Һ������������Ϊ�����ӣ��ټ�������п������ҺpHʹ��Һ��������ת���������˳�ȥ��������Һ����п���Խ�һ��������ҺpH������ԭͭ����Ϊͭ���ʣ����˳�ȥ���õ�����п��Һ������̼�����̼�����ɼ�ʽ̼��п�����ȷֽ����ɻ�������п���Դ˽�������
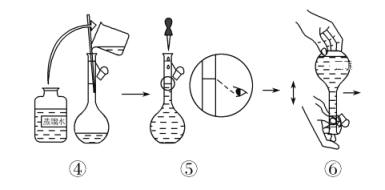
��1��������Һ�巴Ӧʱ�������ı�������Լӿ췴Ӧ���ʣ������¶ȡ��ʵ���������Ũ��Ҳ�ܼӿ췴Ӧ���ʣ�
�ʴ�Ϊ�����Ȼ��ʵ����������Ũ�Ȼ��������
��2������H2O2��Һ������������Ϊ�����ӣ�ͬʱ����ˮ����Ӧ���ӷ���ʽΪ��2Fe2++H2O2+2H+=2Fe3++2H2O��
�ʴ�Ϊ��2Fe2++H2O2+2H+=2Fe3++2H2O��
��3����ZnO����pH��ʹFe3+ת��Ϊ����������������ȥ���������ӳ�����ȫ��ͭ���ӿ�ʼ����pH��֪��������ҺPHΪ4.1��4.7��
�ʴ�Ϊ��4.1��4.7��
��4������III�м���Zn�۽�һ��������ҺpH������ԭͭ����Ϊͭ���ʣ����˳�ȥ��
�ʴ�Ϊ����ȥ��Һ�е�Cu2+��
��5����ʽ̼��п�Ļ�ѧʽΪZn2(OH)2 CO3���ֽ���������п��������̼��ˮ�����ԭ���غ���ƽ��д�õ���Ӧ�Ļ�ѧ����ʽΪ��Zn2(OH)2CO3![]() 2ZnO+CO2��+H2O��
2ZnO+CO2��+H2O��
�ʴ�Ϊ��Zn2(OH)2CO3![]() 2ZnO+CO2��+H2O��
2ZnO+CO2��+H2O��
��6�����ݵζ�ԭ����֪���ζ��յ�ʱ��Һ��ɫ�ɳ�ɫ��Ϊ��ɫ���Ұ���Ӳ���ɫ��Ϊ�ζ��յ���
����H++OH-=H2O��֪�����ᷴӦ��ʣ�����������ʵ���Ϊ0.500mol/L��0.0120L=0.006mol������ZnO��Ӧ�����������ʵ���Ϊ=0.0150L��1.000mol/L��2-0.006mol=0.024mol��
��ZnO+2H+=Zn2++H2O��֪��ZnO������Ϊ0.024mol��![]() ��81g/mol=0.972g��
��81g/mol=0.972g��
����Ʒ����Ϊ![]() ��100%=97.2%��
��100%=97.2%��
�ʴ�Ϊ������Һ�ɳ�ɫ��Ϊ��ɫ�Ұ�����ڲ���ɫ��Ϊ�ζ��յ㣻97.2%��

 ��У����ϵ�д�
��У����ϵ�д�