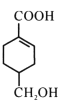
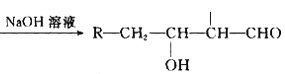��Ŀ����
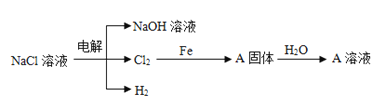
����Ŀ��2019��ŵ������ѧ���䷢������������Ӣ�����ձ�����λ��ѧ�ң���������������ӵ�ط�����о����ס�����ӵ�ع㷺Ӧ��Ҫ����﮵�ط����Խ�Լ��Դ����������������Ӷ��ε����������Ĥ����Ҫ���������(LiCoO2)��Al�ȣ������÷��ϵ�һ�ֹ�������ͼ��ʾ��

�ش��������⣺
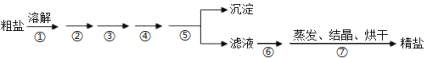
(1)����Ĥ�ڴ���ǰ�������з����Ŀ����________________________��
(2)�ܹ�����������Ч�ʵķ�����________________________(����д����)��
(3)�������ʱAl�ܽ�����ӷ���ʽΪ________________________��
(4)��������ʱ����H2O2��Ŀ����________________________��
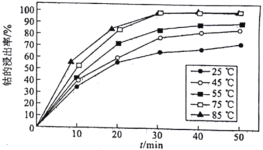
(5)��Һ�¶Ⱥͽ���ʱ����ܵĽ�����Ӱ����ͼ��ʾ����������̵����������_______________________��
(6)����250 mL 1.0 mol/L (NH4)2C2O4��Һ����Ҫ�IJ������������������ձ����Ҫ________��
(7)ȡCoC2O4����4.41g�ڿ����м�����300�����õ��ܵ�������2.41g������ܵ�������Ļ�ѧʽΪ________________________��
���𰸡�����Ӵ�������ӿ췴Ӧ���ʣ���߽����� �ʵ�����NaOH��Ũ�ȡ��ʵ������¶ȵ� ![]() ��LiCoO2��+3�۵�Co��ԭΪ+2�� 75����30min 250mL����ƿ����ͷ�ι� Co3O4
��LiCoO2��+3�۵�Co��ԭΪ+2�� 75����30min 250mL����ƿ����ͷ�ι� Co3O4
��������
�����̿�֪������NaOH��Һ���ݣ���Al����ܽ⣬���˺�õ�����ҺΪ����ƫ�����ƣ�����ΪLiCoO2�������ᡢ�������⣬��LiCoO2��ԭ���õ�����Li+��Co2+����Һ�����백ˮ������ҺpH����ȥ����ӣ�������ҪΪLiOH�����ò�������������õ�CoC2O4���Դ������
(1)����Ĥ�ڴ���ǰ�������з����������Ӵ�������ӿ췴Ӧ���ʣ���߽����ʣ�
(2)�ʵ�����NaOH��Ũ�ȡ��ʵ������¶ȵȶ���������Ч�ʣ�
(3)Al���Ժ�NaOH��Һ��Ӧ����ƫ�����ƺ����������ӷ���ʽΪ![]() ��
��
(4)���ݺ������̿�֪CoԪ����LiCoO2�е�+3�۱�Ϊ��CoC2O4�е�+2�ۣ���˫��ˮ����һ���Ļ�ԭ�ԣ����Լ���˫��ˮ��Ŀ��Ϊ����LiCoO2��+3�۵�Co��ԭΪ+2�ۣ�
(5)��ͼ��֪�¶�Խ���ܵĽ�����Խ��30min��75����85�������½����ʼ�����ͬ��Ϊ�˽����ܺ��¶�Ӧѡ75������30min������ʼ������䣬����ʱ��ѡ30min�����������Ϊ75����30min��
(6)����250 mL 1.0 mol/L (NH4)2C2O4��Һ����Ҫ�IJ������������������ձ��⣬����Ҫ250 mL����ƿ���Լ�����ʱ����Ҫ�Ľ�ͷ�ιܣ�
(7)ȡCoC2O4����4.41 g�ڿ����м�����300�����õ��ܵ�������2.41 g��n(CoC2O4)=![]() =0.03mol������Ԫ���غ㣬��������n(Co)=0.03mol������Ϊm(Co)=0.03mol��59g/mol=1.77g������������m(O)=2.41g-1.77g=0.64g������n(O)=
=0.03mol������Ԫ���غ㣬��������n(Co)=0.03mol������Ϊm(Co)=0.03mol��59g/mol=1.77g������������m(O)=2.41g-1.77g=0.64g������n(O)=![]() =0.04mol����������n(Co)��n(O)=0.03mol��0.04mol=3:4�����Ը�������ΪCo3O4��
=0.04mol����������n(Co)��n(O)=0.03mol��0.04mol=3:4�����Ը�������ΪCo3O4��

����Ŀ���ο�����ͼ�����й�Ҫ��ش����⣺
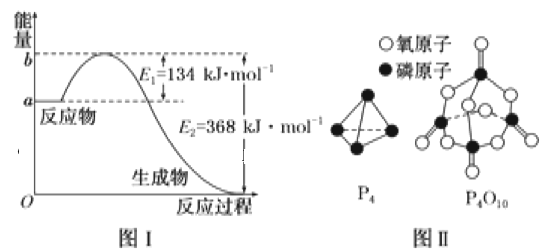
��1��ͼ����1molNO2(g)��1molCO(g)��Ӧ����CO2��NO�����������仯ʾ��ͼ�����ڷ�Ӧ��ϵ�м����������Ӧ��������E2�ı仯��__(�������С�����䡱����ͬ)����H�ı仯��__����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ��__��
��2���±��Dz��ֻ�ѧ���ļ������ݣ�
��ѧ�� | P��P | P��O | O=O | P=O |
����(kJ��mol-1) | 198 | 360 | 498 | x |
��֪1mol����(P4)��ȫȼ�շ���Ϊ1194kJ����������ȫȼ�յIJ���ṹ��ͼ����ʾ�������x=__kJ��mol-1��
��3��PCl5��һ����Ҫ�ĺ���������л��ϳ��������Ȼ�����ij�¶�ʱ����2.0L���º����ܱ������г���1.0molPCl5��������ӦPCl5(g)![]() PCl3(g)+C12(g)��H=+124kJ��mol-1����Ӧ�����вⶨ�IJ������ݼ��±���
PCl3(g)+C12(g)��H=+124kJ��mol-1����Ӧ�����вⶨ�IJ������ݼ��±���
ʱ��t/s | 0 | 50 | 150 | 250 | 350 |
n(PCl3��/mol | 0 | 0.16 | 0.19 | 0.2 | 0.2 |
��Ӧ��ǰ50s��ƽ������v(PCl5)=__���ڸ��¶��£�����ʼʱ����0.5molPCl5��amolCl2��ƽ��ʱPCl5��ת������Ϊ20������a=__��