��Ŀ����
16�� A��B��CΪ������Ԫ�أ������ڱ���������λ������ͼ��ʾ��A��C��Ԫ�ص�ԭ�Ӻ��������֮�͵���Bԭ�ӵ���������B������������������Ӳ���������
A��B��CΪ������Ԫ�أ������ڱ���������λ������ͼ��ʾ��A��C��Ԫ�ص�ԭ�Ӻ��������֮�͵���Bԭ�ӵ���������B������������������Ӳ�����������1��д��Aԭ�ӵ���ʽ
 ��
����2��B��Ԫ�����ڱ��е�λ���ǵ������ڵڢ�A�壮
��3��C��ԭ�ӽṹʾ��ͼΪ
 ��
����4���Ƚ�B��C��ԭ�Ӱ뾶��B��C��д��A����̬�⻯����B������������Ӧ��ˮ���ﷴӦ�Ļ�ѧ����ʽ2NH3+H2SO4�T��NH4��2SO4��
���� A��B��CΪ������Ԫ�أ���A��B��C�����λ�ÿ���A��Cֻ�ܴ��ڵڶ����ڣ���B���ڵ������ڣ���A��ԭ������Ϊx-1����CΪx+1��BΪx+8�����У���x-1��+x+1=x+8��x=8������A��B��C��ԭ�������ֱ�Ϊ7��16��9����Ӧ��Ԫ�طֱ�ΪN��S��F��S��ԭ�Ӱ뾶��ͬ�����OҪ��O��ͬ���ڵ�FҪ����ˣ�S��ԭ�Ӱ뾶����F��ԭ�Ӱ뾶���ݴ˽���С�⼴�ɣ�
��� �⣺��1�������⣬A��B��CΪ������Ԫ�أ���A��B��C�����λ�ÿ���A��Cֻ�ܴ��ڵڶ����ڣ���B���ڵ������ڣ���A��ԭ������Ϊx-1����CΪx+1��BΪx+8�����У���x-1��+x+1=x+8��x=8������A��B��C��ԭ�������ֱ�Ϊ7��16��9����Ӧ��Ԫ�طֱ�ΪN��S��F��N���������5�����ӣ���Nԭ�ӵĵ���ʽΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2��Sλ�ڵ������ڢ�A���壬�ʴ�Ϊ���������ڵڢ�A�壻
��3��Fԭ�Ӻ�����2�����Ӳ㣬�����7�����ӣ�ԭ�ӽṹʾ��ͼΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��4��S��ԭ�Ӱ뾶��ͬ�����OҪ��O��ͬ���ڵ�FҪ����ˣ�S��ԭ�Ӱ뾶����F��ԭ�Ӱ뾶��A����̬�⻯��NH3��B������������Ӧˮ����H2SO4��Ӧ���ɣ�NH4��2SO4���ʴ�Ϊ������2NH3+H2SO4�T��NH4��2SO4��
���� ������Ҫ����Ԫ�����ڱ���λ����ṹ��ϵ���Ӧ�ã���Ϥ���ڱ��Ľṹ�ǽ���Ĺؼ���

| A�� | ��Ӧ��SiO2����������C�ǻ�ԭ�� | |
| B�� | ��Ԫ�ر���ԭ�ˣ�̼Ԫ�ر������� | |
| C�� | �ڷ�Ӧ��C�������������ǻ�ԭ�� | |
| D�� | �ڷ�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ1��1 |
| A�� | HX | B�� | H2X | C�� | XH3 | D�� | XH4 |
| A�� | ����������Ӧ��Ϊ���� | B�� | ����Ϊ���������һ�� | ||
| C�� | �����Խ�ǿ�Ľ���Ϊ���� | D�� | ������ƫ���Ϊ���� |
| A�� | �ȶ��ԣ�HF��HCl��HBr��HI | B�� | �����ԣ�I2��Br2��Cl2 | ||
| C�� | �е㣺H2O��NH3 | D�� | ��ԭ�ԣ�HI��HBr��HCl��HF |
| A�� | H2O��H2O2���ַ�����Oԭ�ӵ��ӻ�������ͬ | |
| B�� | NH3��NH4+�������Ŀռ�ṹ��ͬ | |
| C�� | SO42-��ClO4-�������ļ������ | |
| D�� | SO2��O3���ַ����ǵȵ����� |
| A�� | �������̵ķ�Ӧ�����ɷ�Ӧ�ھ��� | B�� | N2O2��N2O�Ǹ÷�Ӧ�Ĵ��� | ||
| C�� | ��$\frac{1}{2}$v��NO��=v��N2��ʱ����Ӧ�ﵽƽ�� | D�� | �÷�Ӧ�Ļ��Ϊ665kJ•mol-3 |
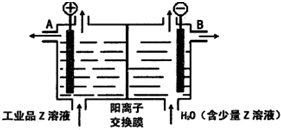 A��B��C��D��E��F����Ԫ��Ϊԭ��������������Ķ�����Ԫ�أ�AΪԭ�Ӱ뾶��С��Ԫ�أ�A��B���γ�4ԭ��10���ӵķ���X��C���������������ڲ��3���� Dԭ�ӵ����������������ڲ��������һ�룻E�ǵؿ��к������Ľ���Ԫ�أ�FԪ�ص��������������۴�����Ϊ6����ش��������⣺
A��B��C��D��E��F����Ԫ��Ϊԭ��������������Ķ�����Ԫ�أ�AΪԭ�Ӱ뾶��С��Ԫ�أ�A��B���γ�4ԭ��10���ӵķ���X��C���������������ڲ��3���� Dԭ�ӵ����������������ڲ��������һ�룻E�ǵؿ��к������Ľ���Ԫ�أ�FԪ�ص��������������۴�����Ϊ6����ش��������⣺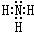 ��D������Һ̬X�з�����������A2C�ķ�Ӧ��д����Ӧ�Ļ�ѧ����ʽ2Na+2NH3=2NaNH2+H2����
��D������Һ̬X�з�����������A2C�ķ�Ӧ��д����Ӧ�Ļ�ѧ����ʽ2Na+2NH3=2NaNH2+H2����