��Ŀ����
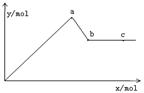
��֪Ba(AlO2)2������ˮ����ͼ��ʾ������Al2(SO4)3��Һ����μ���Ba(OH)2��Һʱ�����ɳ��������ʵ���y�����Ba(OH)2�����ʵ���x�Ĺ�ϵ��
�����й�������ȷ����(����)
A��a��bʱ���������ʵ�����Al(OH)3��BaSO4��
B��c��dʱ��Һ�����ӵ����ʵ�����Ba2����AlO2����
C��a��dʱ���������ʵ�����BaSO4����С��Al(OH)3
D��d��eʱ��Һ�����ӵ����ʵ�����Ba2�����ܵ���OH��
D
����:��a��b��Al3����SO42��ͬʱ������Al3����3OH��===Al(OH)3����Ba2����SO42��===BaSO4������Ba(OH)2����ɿ�֪�������������ʵ���n(BaSO4)>n[Al(OH)3]����b��d�η�����ӦAl(OH)3��OH��===AlO2����2H2O��ÿ����1mol Ba(OH)2�������2 mol AlO2������Bѡ���ȷ����Al2(SO4)3����ɿ�֪������Ӧ������n(BaSO4)>n[Al(OH)3]��C����ȷ������Ba(OH)2��Һ��n(Ba2��)��n(OH��)��1��2��������Ba(AlO2)2��Һ�е���Ba(OH)2ʱ��ʹn(Ba2��)��n(OH��)��

 ��У����ϵ�д�
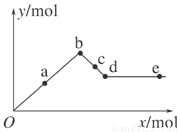
��У����ϵ�д���֪Ba(AlO2)2������ˮ������1 mol Al2(SO4)3����Һ�м��뺬��b mol Ba(OH)2 (b��6)����Һ�����ó��������ʵ���������Ϊ
| A��5mol | B��3mol | C��b/2mol | D��5b/3mol |
��֪Ba(AlO2)2������ˮ����ͼ��ʾ������A12(SO4)3 0.01mol����Һ����μ���Ba(OH)2��Һʱ�����ɳ��������ʵ���y�����Ba(OH)2�����ʵ���x�Ĺ�ϵ������a��c�ֱ���0b�κ�bd�ε��е㣩�������й�������ȷ����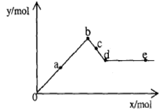
| A��aʱ����������Ϊ3.495 g |
| B��bʱ���������ʵ���Ϊ0.05 mol |
| C��cʱ��Һ��Ba2+���ӵ����ʵ���Ϊ0.005 mol |
| D��eʱ��Һ��AlO2�������ʵ���Ϊ0.01 mol |