��Ŀ����
����Ŀ���±���Ԫ�����ڱ��Ķ����ڲ��֣�������ĸ�ֱ��ʾһ��Ԫ�ء�

��ش��������⣺
��1���ɱ�������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬�������ϡ��Һ�ױ����ֽ⣬��ʹ�õĴ���Ϊ(�����)____��
a.MnO2 b.NaCl c.Na2SO3 d.KMnO4
��2��d��e��h����Ԫ������������Ӧ��ˮ���������������ǿ��˳��Ϊ���û�ѧʽ��ʾ��________��b��c��f��g��h�γɵļ����ӵİ뾶��С�����˳��Ϊ_______(�����ӷ�����д)��
��3����1L���ܱ������У�ͨ��1molf2�����3mola2���壬һ���¶��·�Ӧ����fa3���壬2minʱ�����f2��Ũ��Ϊ0.75mol��L-1����2minʱfa3�����ʵ���Ϊ_____mol����Ӧ�ﵽƽ��״̬ʱ��������fa3�IJ��ʣ����Բ�ȡ��ʩ������ţ�_____��
�������¶� �ں��ݳ��뺤��
�����ܱ������ڳ���f2�� �ܼ�ʱ�����fa3����
��4��d��a���γɻ�����d5a12����д������һ��ȡ������һ�ֵ�ͬ���칹��Ľṹ��ʽ_____��
��5��a��d����Ԫ�ؿ��γɶ��ֻ�������л�����X�IJ����Ǻ���һ������ʯ�ͻ�����չˮƽ�ı�־��X����H2O��һ�������·�Ӧ���ɻ������Ҵ����÷�Ӧ�Ļ�ѧ����ʽΪ��_______��
���𰸡�a H2SiO3��H2CO3��HClO4 Al3+<Na+ <O2-<N3-<Cl- 0.5 �ۢ� C��CH3��4 CH2=CH2+H2O![]() CH3CH2OH
CH3CH2OH
��������
��Ԫ�����ڱ����λ�ÿ�֪��Ԫ��a��h�ֱ�ΪH��Na��Al��C��Si��N��O��Cl��
��1����������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬�������ϡ��Һ�ױ����ֽ⣬����Һ�ǹ���������Һ���������̿�����������ֽⷴӦ�Ĵ�������ѡa��
��2���ǽ�����������Խǿ������ۺ����������Խ��H2SiO3��H2CO3��HClO4�����ӵĺ�����Ӳ���Խ�࣬���Ӱ뾶Խ��������Ų���ͬ�����������뾶��ԭ���������������С����Na��Al��N��O��Cl�γɵļ����ӵİ뾶��С�����˳��ΪAl3+<Na+ <O2-<N3-<Cl-��
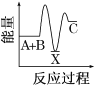
��3��������Ӧ��N2+3H2 ![]() 2NH3��2minʱ�����N2��Ũ��Ϊ0.75mol��L-1����N2�����ʵ����仯��1 mol ��0.75mol =0.25mol�������ʵ����仯��֮�ȵ��ڻ�ѧ������֮�ȵã�2minʱNH3�����ʵ���Ϊ0.5 mol��
2NH3��2minʱ�����N2��Ũ��Ϊ0.75mol��L-1����N2�����ʵ����仯��1 mol ��0.75mol =0.25mol�������ʵ����仯��֮�ȵ��ڻ�ѧ������֮�ȵã�2minʱNH3�����ʵ���Ϊ0.5 mol��
�ٸ÷�Ӧ���ȣ������¶ȣ�ƽ�������ƶ��� NH3�IJ��ʽ��ͣ��ʢٴ���
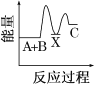
�ں��ݳ��뺤����ƽ�ⲻ�����ƶ��� NH3�IJ��ʲ��䣬�ʢڴ���
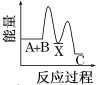
�����ܱ������ڳ���N2��ƽ�������ƶ��� NH3�IJ������ʢ���ȷ��
�ܼ�ʱ�����NH3��ƽ�������ƶ��� NH3�IJ������ʢ���ȷ��
��ѡ�ۢܣ�
��4��C5H12������һ��ȡ������һ�ֵ�ͬ���칹���������飬��ṹ��ʽΪC��CH3��4��
��5��C2H4�IJ����Ǻ���һ������ʯ�ͻ�����չˮƽ�ı�־��C2H4����H2O��һ�������·�Ӧ���ɻ������Ҵ����÷�Ӧ�Ļ�ѧ����ʽΪCH2=CH2+H2O![]() CH3CH2OH��
CH3CH2OH��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�