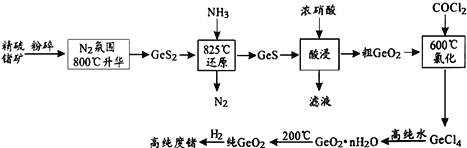
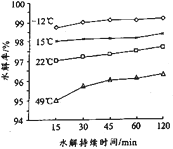
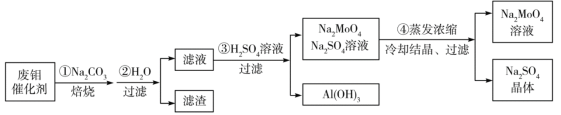
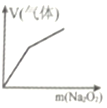
��Ŀ����
����Ŀ�����������⣩�о���ѧϰС���е�С��ͬѧ��ѧϰ�з��֣�ͨ������CO2�ñ���ʯ��ˮ������CO2��ŨNaOH��Һ��
��ʵ��̽�����������ͬʢ��CO2��������ƿ�У��ֱ���������ı���ʯ��ˮ��ŨNaOH��Һ��ʵ��װ�ú�������ͼ��ʾ������һ����롣
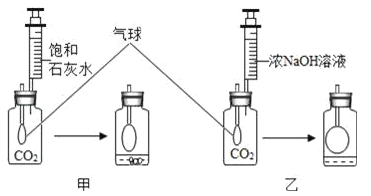
��������ۣ���װ���в�����ʵ������Ļ�ѧ����ʽΪ______________________������ͨ����ʯ��ˮ������NaOH��Һ����CO2��ԭ��________________________________________________����װ���е�ʵ��������___________________________������CO2�϶��װ����__________________��
��������֤����һλͬѧС��ͨ�����㷢�֣���������Ca(OH)2��NaOH����CO2��������Ca(OH)2����NaOH����ˣ�����Ϊͨ������CO2Ӧ���ñ���ʯ��ˮ��
��˼�����ۣ������С��ͬѧ�Ľ��۽������ۣ�________________________________________________________��
���𰸡�CO2��Ca(OH)2��CaCO3����H2O��CO2������ CO2��ʯ��ˮ���������Ե�����NaOH�������������� �������������Һ������� �� ��ΪCa(OH)2���ܽ�Ƚ�С�����γɵı���ʯ��ˮ�����ʵ�����������С
��������
��װ���У�����ʯ��ˮ�е�Ca(OH)2��CO2��Ӧ������CaCO3��ɫ�������ɴ˿�д����Ӧ�ķ���ʽ��NaOH��Һ����CO2��������������װ���У�����CO2��NaOH���գ����¹��ƿ�������ѹǿ��С�����ǿ������������ڣ��ɴ˿���֪ʵ��������ƿ����Һ���������ʵ����ʵ�������Ӧ����ʽ�����ۺϷ�������ȷ����ѡװ�á�
С�����ͬ���ļ�������������ǣ���������Һ��Ũ�ȡ�
��װ���У�����ʯ��ˮ�е�����ΪCa(OH)2������CO2��Ӧ�Ļ�ѧ����ʽΪCO2��Ca(OH)2��CaCO3����H2O��CO2��������ͨ����ʯ��ˮ������NaOH��Һ����CO2������ΪCO2��ʯ��ˮ���������Ե�����NaOH����������������װ���У�����CO2��NaOH���գ����¹��ƿ�������ѹǿ��С�����ǿ������������ڣ��Ӷ������������������Һ������ǡ���Ϊ����ʯ��ˮ��Ca(OH)2����ˮ��Ũ�Ⱥ�С�����ʵ����ʵ�����С����������CO2�϶��װ�����ҡ���Ϊ��CO2��Ca(OH)2��CaCO3����H2O��CO2��������CO2��ʯ��ˮ���������Ե�����NaOH���������������������������Һ������ǣ��ң�
С�����ͬ���ļ�������������ǣ���������Һ��Ũ�ȡ���ΪCa(OH)2���ܽ�Ƚ�С�����γɵı���ʯ��ˮ�����ʵ�����������С����Ϊ����ΪCa(OH)2���ܽ�Ƚ�С�����γɵı���ʯ��ˮ�����ʵ�����������С��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��A��B��C��D��EΪԭ��������������Ķ���������Ԫ�أ���ԭ�Ӱ뾶�������������Ĺ�ϵ����ͼ1��Eԭ��������ϵĵ�������Dԭ��������������4����D���Ӻ�������Ų���C2����ͬ��

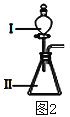
�Իش�
��1��Ԫ��E��Ԫ�����ڱ��е�λ����___________________��
��2����Ԫ��D��������������������������ͬ������________________�����û�ѧʽ������ͬ��
��3��B��E����������Ӧ��ˮ������Խ�����_________________��������ͼ2��װ����֤�������������ǿ��������װ���м�����Լ��ֱ�Ϊ����___________����__________���۲쵽��ʵ��������________________________��
��4������������Ԫ����ɵ����ʣ�����ɺͽṹ��Ϣ���±���
���� | ��ɺͽṹ��Ϣ |
a | ����A��C��D���� |
b | C��D��ɵĻ������ԭ����֮��Ϊ1��1 |
c | ��ѧ���ΪAC2 |
��a���еĻ�ѧ����___________________________________��
��b��c��Ӧ�Ļ�ѧ����ʽΪ_________________________________��
����Ŀ������ʵ����ͼ��Ӧ����
ѡ�� | A | B | C | D |
ʵ�� | ��NH4C1��Һ�м������Na2O2���� | ��ϡ������Һ�м������������ | ������Һ����μ�Ba��OH��2��Һ������ | �����ʵ�����NaOH��Na2CO3�Ļ����Һ�еμӹ�����ϡ���� |
ͼ�� |
|
|
|
|
A.AB.BC.CD.D
����Ŀ����֪��pAg��-lgc(Ag+)��pX��-lg c(Xn-)��298Kʱ���������ʵ�Ksp���±���
��ѧʽ | AgCl | AgSCN | Ag2CrO4 |
��ɫ | �� | dz�� | �� |
Ksp | 1.8��1010 | 1.0��1012 | 2.0��1012 |
AgCl��AgSCN��Ag2CrO4�ı�����Һ�У������Ӻ������ӵ�Ũ�ȹ�ϵ��ͼ��ʾ������˵����ȷ����
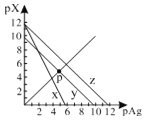
A.ͼ��x����AgCl���ߣ�z����AgSCN����
B.298Kʱ����Cl����CrO42�������ʵ���Ũ�Ⱦ�Ϊ0.1mol/L����Һ�У���������0.1mol/L��AgNO3��Һ�����Ȳ������Ǻ�ɫ����
C.298Kʱ������p���������Ũ�ȣ���y�ϵĵ������������ƶ�
D.298KʱAg2CrO4(s)��2SCN��(aq) ![]() 2AgSCN(s)��CrO42- (aq)��ƽ�ⳣ��K��2.0��1012
2AgSCN(s)��CrO42- (aq)��ƽ�ⳣ��K��2.0��1012