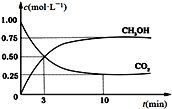
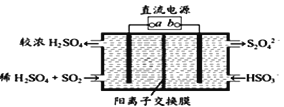
��Ŀ����
����Ŀ��ijѧϰС������ͼ��ʾװ�òⶨ��þ�Ͻ������������������������ԭ��������
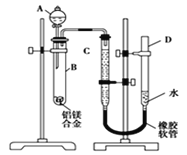
(1)A���Լ�Ϊ________��
(2)ʵ��ǰ���Ƚ���þ�Ͻ���ϡ���н���Ƭ�̣���Ŀ����__________________________
(3)��������ԣ���ҩƷ��ˮװ��������У����Ӻ�װ�ú�����еIJ������У��ټ�¼C��Һ��λ�ã��ڽ�B��ʣ�������ˣ�ϴ�ӣ�������أ��۴�B�в���������������ָ������º�¼C��Һ��λ�ã�����A��B�еμ������Լ�������������˳����____________(�����)����¼C��Һ��λ��ʱ����ƽ���⣬��Ӧ____________________________��
(4)ʵ������У���δϴ�ӹ������õIJ����������������������________(����ƫ������ƫС����������Ӱ����)��
(5)ʵ������Ҫ��480 mL 1 mol/L�����ᣬ���ƹ��������ڶ��ݵIJ��������ǽ�ͷ�ιܡ���������_____________________(��������������)
���𰸡�NaOH��Һ ��ȥ��þ�Ͻ���������Ĥ �٢ܢۢ� ʹD��C��Һ����ƽ ƫС 500 mL����ƿ
��������
����ʵ��Ŀ��ԭ������ʵ��ѡ���ҩƷ�������е�ע���������ʵ��ԭ����ʵ����������������������Һ����ԭ������ʵ��������������
(1)þ��������ǿ�ᷴӦ��������������Ҫ�����������������ѡ�õ��Լ�������������Һ����Ϊþ������������Һ����Ӧ��
(2)þ�������Ƚϻ��ã��ڿ��������ױ������γ�����Ĥ�����Խ��ݵ�Ŀ���dz�ȥ��þ�Ͻ���������Ĥ��
(3)ʵ��ʱͨ����Ʒ������þ����������Al��������Al�����������������Ͻ��������������Ʒ�Ӧ���ɵ��������������������������ԭ�����������Ը���ʵ��ԭ������ʵ�鲽��Ϊ������¼C��Һ��λ�ã�����A��B�еμ������Լ�������B�в���������������ָ������º�¼C��Һ��λ�ã�����B��ʣ�������ˣ�ϴ�ӣ�������أ�������������˳�����٢ܢۢ� ��Ϊ���ų�ѹǿ�����������Ӱ�죬�ڼ�¼C��Һ��λ��ʱ����ƽ���⣬��ӦʹD��C��Һ����ƽ��
(4)��δϴ�ӹ������õIJ����ʹ�ò�����þ������ƫ��������������������ƫС��
(5)ʵ������Ҫ��480 mL 1 mol/L�����ᣬ���ݡ����������ԭ��Ӧ����500mL��Һ�����ݹ����л���Ҫ��������500 mL����ƿ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�