��Ŀ����
ʵ����������0.5mol?L-1��NaOH��Һ500mL�����������������ձ� ��100mL��Ͳ ��1000mL ����ƿ ��500mL ����ƿ �ݲ����� ��������ƽ�������룩
��1������ʱ������ʹ�õ�������______������ţ�����ȱ�ٵ�������______��ʵ���������õ��������������÷ֱ���______��______��
��2��ʹ������ƿǰ������е�һ��������______��
��3������ʱ��һ��ɷ�Ϊ���¼������裺�ٳ��� �ڼ��� ���ܽ� ��ҡ�� ��ת�� ��ϴ�� �߶��� ����ȴ������ȷ�IJ���˳����______��
���𰸡���������1������ʵ���������ȷ��ÿ��������Ҫ��������;��
��2������ƿʹ��֮ǰӦ�������Ƿ�©ˮ��
��3����������Һ��ʵ��������̽���ʵ�鲽������
����⣺��1������0.5mol?L-1��NaOH��Һ500mL�����Ʋ����г������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ���������ƽ����NaOH���õ�ҩ�ף������ձ���ϡ�ͣ����ò��������裬�����ܽ⣬��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ��2-3�Σ�����ϴ��Һ��������ƿ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�
���Ը�������������ʹ�õ������Т٢ܢݢޣ�����Ҫҩ�ס���ͷ�ιܣ�ʵ���������õ��������������÷ֱ��ǽ��衢������
�ʴ�Ϊ���٢ܢݢޣ�ҩ�ס���ͷ�ιܣ����衢������
��2������ƿʹ��֮ǰӦ�������Ƿ�©ˮ��
�ʴ�Ϊ����©��
��3������0.5mol?L-1��NaOH��Һ500mL�����Ʋ����м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ���������ƽ����NaOH���õ�ҩ�ף������ձ���ϡ�ͣ����ò��������裬�����ܽ⣬��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ��2-3�Σ�����ϴ��Һ��������ƿ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�
���Բ���˳���Ǣڢ٢ۢ�ݢޢߢܣ�
�ʴ�Ϊ���ڢ٢ۢ�ݢޢߢܣ�
���������⿼����һ�����ʵ���Ũ����Һ�����ƣ�ע���c=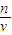 ��������ԭ����ע���������Ƶij���Ӧ����С�ձ��ܣ�
��������ԭ����ע���������Ƶij���Ӧ����С�ձ��ܣ�
��2������ƿʹ��֮ǰӦ�������Ƿ�©ˮ��
��3����������Һ��ʵ��������̽���ʵ�鲽������
����⣺��1������0.5mol?L-1��NaOH��Һ500mL�����Ʋ����г������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ���������ƽ����NaOH���õ�ҩ�ף������ձ���ϡ�ͣ����ò��������裬�����ܽ⣬��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ��2-3�Σ�����ϴ��Һ��������ƿ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�
���Ը�������������ʹ�õ������Т٢ܢݢޣ�����Ҫҩ�ס���ͷ�ιܣ�ʵ���������õ��������������÷ֱ��ǽ��衢������
�ʴ�Ϊ���٢ܢݢޣ�ҩ�ס���ͷ�ιܣ����衢������
��2������ƿʹ��֮ǰӦ�������Ƿ�©ˮ��
�ʴ�Ϊ����©��
��3������0.5mol?L-1��NaOH��Һ500mL�����Ʋ����м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ���������ƽ����NaOH���õ�ҩ�ף������ձ���ϡ�ͣ����ò��������裬�����ܽ⣬��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ��2-3�Σ�����ϴ��Һ��������ƿ������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�
���Բ���˳���Ǣڢ٢ۢ�ݢޢߢܣ�
�ʴ�Ϊ���ڢ٢ۢ�ݢޢߢܣ�
���������⿼����һ�����ʵ���Ũ����Һ�����ƣ�ע���c=
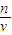 ��������ԭ����ע���������Ƶij���Ӧ����С�ձ��ܣ�
��������ԭ����ע���������Ƶij���Ӧ����С�ձ��ܣ� 
��ϰ��ϵ�д�
 ���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
�����Ŀ

 ʵ����������0.5mol/LNaOH��Һ500mL��
ʵ����������0.5mol/LNaOH��Һ500mL��