��Ŀ����
��16�֣����������Ҫ����������������(�׳��������)���������������ʡ����й�������(CaO2)���ճ������ũҵ�����л�����������������ҩ�����졢��֬Ư����������ȣ���������Ϊ��������������֪������������CO2��Ӧ���������ɣ�����SO2ͨ��������Ʒ�ĩ��Ҳ���������ɡ��������CO2��SO2��������Ƶķ�Ӧԭ����ͬ����Ҳ�������SO2���н�ǿ�Ļ�ԭ�ԣ�CO2��ǿ��ԭ�ԣ���Ӧԭ������ͬ���ݴ��������ʵ����������жϡ�
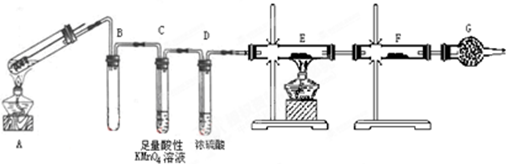
ʵ��һ��ͨ���������������жϷ����Ļ�ѧ��Ӧ��ʵ��װ�����£�
(1)�Լ�A����ѡ��________���Լ�B��������________��
(2)װ��E���ڲⶨ��������������ڷ����л���װ��ͼ��
(3)ʵ����װ��C�й�����������������m1 g��װ��D����������m2 g��װ��E���ռ���������ΪV L(�ѻ���ɱ�״����)���������йز��������жϣ�SO2δ�����������ֱ���������ȫ��������V����m1��ϵʽ��
δ��������____________�����ֱ�������____________��
��ȫ��������____________��
��SO2��ȫ��������д����Ӧ�Ļ�ѧ����ʽ��___________________________��
ʵ�������һ�����Ĺ������ƹ�����ͨ��������SO2��ȡ��Ӧ��Ĺ������ʵ��̽������֤������������SO2��Ӧ���ص㡣
������裺
����1����Ӧ�����ֻ��________��֤��SO2δ��������
����2����Ӧ�������ֻ��________��֤��SO2��ȫ��������
����3��________________________________________________________��
ʵ��̽����
(4)���ʵ�飬֤������3����ȷ�ģ���Ҫ�ش�ʵ����̡�����ͽ��ۣ�______
_____________________________________________________________��
ʵ�����ۣ�
(5)ʵ�������SO2��ͨ����ֱ��Ӱ��̽��ʵ�����Ŀ�ѧ�ԣ����Ҫ˵��ԭ��___________________________________________________________��
ʵ��һ��ͨ���������������жϷ����Ļ�ѧ��Ӧ��ʵ��װ�����£�

(1)�Լ�A����ѡ��________���Լ�B��������________��
(2)װ��E���ڲⶨ��������������ڷ����л���װ��ͼ��
(3)ʵ����װ��C�й�����������������m1 g��װ��D����������m2 g��װ��E���ռ���������ΪV L(�ѻ���ɱ�״����)���������йز��������жϣ�SO2δ�����������ֱ���������ȫ��������V����m1��ϵʽ��
δ��������____________�����ֱ�������____________��
��ȫ��������____________��
��SO2��ȫ��������д����Ӧ�Ļ�ѧ����ʽ��___________________________��
ʵ�������һ�����Ĺ������ƹ�����ͨ��������SO2��ȡ��Ӧ��Ĺ������ʵ��̽������֤������������SO2��Ӧ���ص㡣
������裺
����1����Ӧ�����ֻ��________��֤��SO2δ��������
����2����Ӧ�������ֻ��________��֤��SO2��ȫ��������
����3��________________________________________________________��
ʵ��̽����
(4)���ʵ�飬֤������3����ȷ�ģ���Ҫ�ش�ʵ����̡�����ͽ��ۣ�______
_____________________________________________________________��
ʵ�����ۣ�
(5)ʵ�������SO2��ͨ����ֱ��Ӱ��̽��ʵ�����Ŀ�ѧ�ԣ����Ҫ˵��ԭ��___________________________________________________________��
(1)Ũ���ᡡ����δ��Ӧ��SO2 (��һ��)
(2) (���������𰸾���)��2�֣�
(���������𰸾���)��2�֣�
(3)V=7m1/30��0��V��7m1/30��V=0 CaO2+SO2===CaSO4��5�֣�
����1��Na2SO3������2��Na2SO4������3������ΪNa2SO3��Na2SO4�Ļ���֤���������ֱ�������3�֣�
(4)ȡ��Ӧ��Ĺ�������Թ��У�������ˮ�ܽ⣬����BaCl2��Һ�а�ɫ�����������ټ���ϡ���ᣬ���������ܽ⣬֤��ԭ�����м�����������Ҳ�������ƣ���Na2O2��SO2����������2�֣�
(5)��CaO2��ʣ�࣬�����ˮ�ܽ�ʱ�����������ܽ�SO32-������SO�������SOʱ����˵�����ǹ���������SO2��Ӧ���ɵģ�2�֣�
(2)
 (���������𰸾���)��2�֣�
(���������𰸾���)��2�֣�(3)V=7m1/30��0��V��7m1/30��V=0 CaO2+SO2===CaSO4��5�֣�
����1��Na2SO3������2��Na2SO4������3������ΪNa2SO3��Na2SO4�Ļ���֤���������ֱ�������3�֣�
(4)ȡ��Ӧ��Ĺ�������Թ��У�������ˮ�ܽ⣬����BaCl2��Һ�а�ɫ�����������ټ���ϡ���ᣬ���������ܽ⣬֤��ԭ�����м�����������Ҳ�������ƣ���Na2O2��SO2����������2�֣�
(5)��CaO2��ʣ�࣬�����ˮ�ܽ�ʱ�����������ܽ�SO32-������SO�������SOʱ����˵�����ǹ���������SO2��Ӧ���ɵģ�2�֣�
��1������װ�ÿ�֪��A����ȡSO2�ġ����������ɵ�SO2�к���ˮ��������ˮ����Ҳ�ܺ������Ʒ�Ӧ������װ��B�е��Լ�Ӧ����Ũ���ᣬ��������SO2������SO2�������Ƶķ�Ӧ�У�SO2�ǹ����ģ�����Ϊ�˷�ֹSO2�Է�Ӧ�����ɵ�����ĸ��ţ���װ��D��Ӧ��ʢ�ż�ʯ�ң���������δ��Ӧ��SO2��
��2����������������ˮ������Ӧ������ˮ����������������������װ��ͼ�ǣ����𰸣���
��3�����SO2û�б���������Ӧʽ��2CaO2��2SO2=2CaSO3��O2���������ɵ����������V��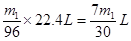 ���������ȫ��������������������0������Ӧ�ķ���ʽΪCaO2+SO2===CaSO4���������Dz��ֱ������������ɵ�������0��V��7m1/30���������Ϸ�����֪��������ɵĹ�����ֻ���������ƣ���SO2δ��������������ɵĹ���ֻ�������ƣ���ȫ������������������������ƣ�Ҳ�������ƣ���SO2�Dz��ֱ�������
���������ȫ��������������������0������Ӧ�ķ���ʽΪCaO2+SO2===CaSO4���������Dz��ֱ������������ɵ�������0��V��7m1/30���������Ϸ�����֪��������ɵĹ�����ֻ���������ƣ���SO2δ��������������ɵĹ���ֻ�������ƣ���ȫ������������������������ƣ�Ҳ�������ƣ���SO2�Dz��ֱ�������
��4��Ҫ֤������3��ȷ������������������ƺ������Ƶ����ʲ��죬��ȡ��Ӧ��Ĺ�������Թ��У�������ˮ�ܽ⣬����BaCl2��Һ�а�ɫ�����������ټ���ϡ���ᣬ���������ܽ⣬֤��ԭ�����м�����������Ҳ�������ƣ���Na2O2��SO2����������
��5�����ڹ������ƾ��������ԣ�����������������ʣ�࣬�����ˮ�ܽ�ʱ�����������ܽ�SO32-������SO�������SOʱ����˵�����ǹ���������SO2��Ӧ���ɵġ�
��2����������������ˮ������Ӧ������ˮ����������������������װ��ͼ�ǣ����𰸣���
��3�����SO2û�б���������Ӧʽ��2CaO2��2SO2=2CaSO3��O2���������ɵ����������V��
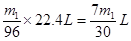 ���������ȫ��������������������0������Ӧ�ķ���ʽΪCaO2+SO2===CaSO4���������Dz��ֱ������������ɵ�������0��V��7m1/30���������Ϸ�����֪��������ɵĹ�����ֻ���������ƣ���SO2δ��������������ɵĹ���ֻ�������ƣ���ȫ������������������������ƣ�Ҳ�������ƣ���SO2�Dz��ֱ�������
���������ȫ��������������������0������Ӧ�ķ���ʽΪCaO2+SO2===CaSO4���������Dz��ֱ������������ɵ�������0��V��7m1/30���������Ϸ�����֪��������ɵĹ�����ֻ���������ƣ���SO2δ��������������ɵĹ���ֻ�������ƣ���ȫ������������������������ƣ�Ҳ�������ƣ���SO2�Dz��ֱ���������4��Ҫ֤������3��ȷ������������������ƺ������Ƶ����ʲ��죬��ȡ��Ӧ��Ĺ�������Թ��У�������ˮ�ܽ⣬����BaCl2��Һ�а�ɫ�����������ټ���ϡ���ᣬ���������ܽ⣬֤��ԭ�����м�����������Ҳ�������ƣ���Na2O2��SO2����������
��5�����ڹ������ƾ��������ԣ�����������������ʣ�࣬�����ˮ�ܽ�ʱ�����������ܽ�SO32-������SO�������SOʱ����˵�����ǹ���������SO2��Ӧ���ɵġ�

��ϰ��ϵ�д�
�����Ŀ