��Ŀ����
����Ŀ��CO��H2�ǹ�ҵ����õĺϳ������úϳ������Ʊ������ܶ࣬����Ҳ�ܺϳ�������Ҫ���л���ش��������⣺
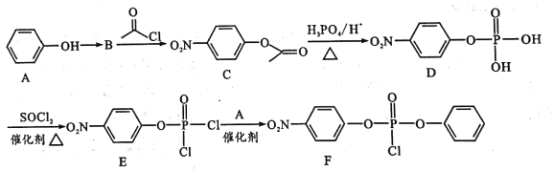
(1)�Ʊ��úϳ�����һ�ַ�������CH4��H2OΪԭ�ϣ��йط�Ӧ�������仯��ͼ��ʾ��
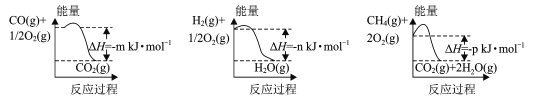
CH4 (g)��H2O(g)��Ӧ����CO(g)��H2 (g)���Ȼ�ѧ����ʽΪ____��
(2)��ҵ�Ҵ�Ҳ����CO��H2�ϳɣ�����һ�����״��������Ͻ�ʹ�óɱ������Ĺ�ҵ�ƾ�����ʳ�þƣ���һ�㶨�Եķ������Ѽ���ʳ�þ��еļ״������˾��������ữ�ij�ɫK2Cr2O7��Һ�����ⶨ������м״��ĺ������״�������K2Cr2O7��Һ��Ӧ����CO2��Cr2(SO4)3�����ʣ�д���仯ѧ����ʽ ___________��
(3)Ϊ�˼�����CO��H2�ϳ����ϳɵ�ij�л���M����ɣ����������²ⶨ����1.84gM�������г��ȼ�գ������ɵ���������ͨ�������ļ�ʯ�ң���ʯ�� ����4. 08 g����֪����CO2��H2O�����ʵ���֮��Ϊ3:4����M��̼���⡢��ԭ�Ӹ���֮��Ϊ____��
(4) CO2��H2�ϳɼ״��漰���·�Ӧ��CO2(g)+3H2(g)CH3OH(g)+H2O(g) H=-49.58kJ/mol���ڷ�Ӧ�����п����ں�ѹ���ܱ������У�����һ������CO2��H2����ò�ͬ�¶��£���ϵ��CO2��ƽ��ת������ѹǿ�Ĺ�ϵ������ͼ��ʾ��
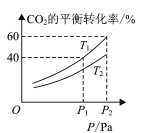
�ٷ�Ӧ�����У�������Ӧ�ﵽƽ��״̬�ı�־��______;
A.����3mol O-H����ͬʱ����3mol H-H�� B.�����������ѹǿ���ٱ仯
C.�����������ƽ��Ħ���������� D.CH3OH��Ũ�Ȳ��ٸı�
�ڱȽ�T1��T2�Ĵ�С��ϵ��T1 ___T2 (����<������=������>��)��
����T1��P2�������£����ܱ������г���1mol CO2��3mol H2���÷�Ӧ�ڵ�5 minʱ�ﵽƽ�⣬��ʱ�������ݻ�Ϊ2.4 L����÷�Ӧ�ڴ��¶��µ�ƽ�ⳣ��Ϊ____������T1�ʹ�ʱ�������ݻ����䣬�ٳ���1mol CO2��3mol H2����ﵽƽ��ʱCO2����ת����Ϊa��д��һ���ܹ����a�ķ��̻�ʽ�� ___(���ػ����Բ�����λ)��
���𰸡�CH4(g)+H2O(g)=CO(g)+3H2(g) ��H=+(-p+3n+m)kJ��mol��1 CH3OH+K2Cr2O7+4H2SO4=CO2+K2SO4+Cr2(SO4)3+6H2O 3:8:3 CD �� 3 
��������
(1)����ͼ����д�Ȼ�ѧ����ʽ����ϸ�˹���ɼ��㷴Ӧ�ȣ�
(2)����ṩ��Ϣ������������ԭ��Ӧ����غ�������غ���д����ʽ��
(3)�����л���ȼ��̼ԭ���غ㡢��ԭ���غ㣬�Լ�������ı�����ϵ�з��̼���M�е�̼���⡢����Ŀ��
(4)�ٸ���ƽ��״̬���ص㣬�Լ�������ɲ�����ʱ��Ӧ�ﵽƽ��ȷ����жϣ�
�ڶ�����̼��ת�������¶ȵ����߶����ͣ��ݴ˷�����
���С�����ʽ���������¶Ȳ��䣬ƽ�ⳣ������Ĺ�ϵ���н����㡣
(1)��������������ϵͼ��д����Ӧ���Ȼ�ѧ����ʽ��CO(g)��![]() O2(g)= CO2(g) ��H1����m kJ��mol��1��H2(g)��
O2(g)= CO2(g) ��H1����m kJ��mol��1��H2(g)��![]() O2(g)=H2O(g) ��H2����n kJ��mol��1��CH4(g)��2O2(g)=CO2(g)+ 2H2O(g)��H3����p kJ��mol��1���ɸ�˹���ɵã�CH4(g)+H2O(g)=CO(g)+3H2(g)��H����H3��3��H2����H1��+(��p+3��n+m)kJ��mol��1��
O2(g)=H2O(g) ��H2����n kJ��mol��1��CH4(g)��2O2(g)=CO2(g)+ 2H2O(g)��H3����p kJ��mol��1���ɸ�˹���ɵã�CH4(g)+H2O(g)=CO(g)+3H2(g)��H����H3��3��H2����H1��+(��p+3��n+m)kJ��mol��1��
(2)�״�������K2Cr2O7��Һ��Ӧ����CO2��Cr2(SO4)3��Cr�Ļ��ϼ۽���3��̼�Ļ��ϼ�����6�����ݵ���غ㣬�����غ�ɵ�CH3OH+K2Cr2O7+4H2SO4=CO2+K2SO4+Cr2(SO4)3+6H2O��
(3)����1.84g M�к�Cx mol����H y mol���������� �����x=0.06 y=0.16����1.84g M�к������ʵ���z=
�����x=0.06 y=0.16����1.84g M�к������ʵ���z=![]() =0.06 mol��1mol M�к�C��H��O�ֱ�Ϊ3��8��3����M��̼���⡢��ԭ�Ӹ���֮��Ϊ3:8:3��
=0.06 mol��1mol M�к�C��H��O�ֱ�Ϊ3��8��3����M��̼���⡢��ԭ�Ӹ���֮��Ϊ3:8:3��
(4)��A������3 mol H��H������ζ��������3molH2��CH3OH��H2O�о���H��O��������Ӧ������3mol O-H��������������Ӧ���ʵı仯��ϵ�����Բ���˵��v��=v������A����
B�����ڷ�Ӧ������������ѹǿһֱ���ֲ��䣬����ѹǿ���ٱ�����ʱ��Ҳ����˵��v��=v������B����
C�������з�Ӧ��������Ϊ���壬����������mһֱ���ֲ��䣬����Ӧǰ���������ʵ������n��0����ֻ�е���Ӧ�ﵽƽ��״̬ʱ����ϵ�������ʵ���n�Ų��ٱ仯������M=![]() ֪��ƽ��Ħ�������������ܹ�������Ӧ�ﵽƽ��״̬����C��ȷ��
֪��ƽ��Ħ�������������ܹ�������Ӧ�ﵽƽ��״̬����C��ȷ��
D����CH3OH��Ũ�Ȳ��ٸı�����������λʱ�������ɺ����ĵ�CH3OH��Ũ����ȣ���v��=v������Ӧ�ﵽƽ��״̬����D��ȷ��
��ѡCD��
������H����49.58 kJ��mol��1��0��֪����ӦΪ���ȷ�Ӧ����ͬһѹǿ�£��¶����߿�ʹCO2��ת���ʽ��ͣ�����ͼ���֪T1��T2��
����ͼ1֪����T1��p2�������£�ƽ��ʱCO2��ת����Ϊ60%�����ĵ�CO2Ϊ1mol ��60%��0.6 mol���������¹�ϵ��

������������(2.4L)�У�ƽ����ϵ�и����ʵ�Ũ��Ϊc(CO2)=![]() mol��L��1��c(H2)=
mol��L��1��c(H2)=![]() mol��L��1��c(CH3OH)=
mol��L��1��c(CH3OH)=![]() mol��L��1��c(H2O)=
mol��L��1��c(H2O)=![]() mol��L��1����������µ�ƽ�ⳣ��Ϊ
mol��L��1����������µ�ƽ�ⳣ��Ϊ �������ٳ���ԭ����ʱ����Ӧ�¶�û�䣬��K���䡣��ﵽƽ��ʱCO2����ת����Ϊa�������������з�Ӧ��CO2Ϊ2a mol��ͬ�������¹�ϵ��
�������ٳ���ԭ����ʱ����Ӧ�¶�û�䣬��K���䡣��ﵽƽ��ʱCO2����ת����Ϊa�������������з�Ӧ��CO2Ϊ2a mol��ͬ�������¹�ϵ��

 ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ���������ȣ�ClO2����һ�ָ�Ч��������������ˮ���е�Ϊ11.0�������ױ�ը���ڸ������ϡ�������£��ø����������������������Ʊ��������ȣ�װ����ͼ��
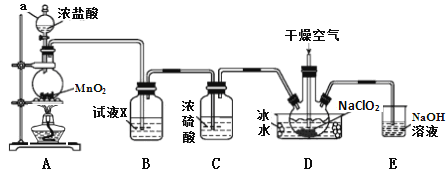
(1)����a������Ϊ_____________��װ��A�з�Ӧ�����ӷ���ʽΪ_______________��
(2)�Լ�X��_______________________��
(3)װ��D�б�ˮ����Ҫ������___________��װ��D�ڷ�����Ӧ�Ļ�ѧ����ʽΪ_______________��
(4)װ��E����Ҫ��Ӧ�����ӷ���ʽΪ��____________________________��
(5)��֪NaClO2������Һ�ڲ�ͬ�¶�ʱ�����ľ���������±���
�¶� | ��38�� | 38����60�� | ��60�� |
�������� | NaClO2��3H2O | NaClO2 | �ֽ��NaClO3��NaCl |
����NaClO2��Һ�Ƶ�NaClO2����IJ������裺 55�������ᾧ��_________��38��60������ˮϴ�ӡ�����60�����
(6)��ҵ��Ҳ�������·����Ʊ�ClO2��
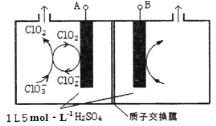
������������˫��ˮ��NaClO3��Ӧ����Ӧ�����ӷ���ʽΪ_______________________��
����ͼ��ʾΪֱ�ӵ�������ơ��Զ���ѭ���Ʊ��ߴ�ClO2��ʵ�顣�������缫��ӦʽΪ____________��