��Ŀ����
| �±��Dz��ֶ�����Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼ� |
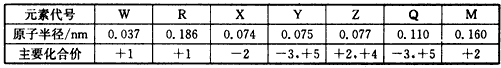 |
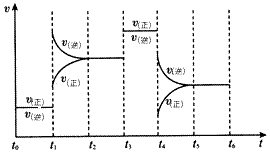
| �Իش��������⣺ (1)����Ԫ����ԭ�Ӱ뾶����Ԫ�������ڱ���λ����___________��M��Z�������������ȼ�յĻ�ѧ����ʽΪ________________�� (2)X����R��1��1��ԭ�Ӹ������γɻ�����ף����д��ڵĻ�ѧ����____________��X����W��ɺ�18���ӵĻ������ң����ҵĵ���ʽΪ___________�� (3)M������������ˮ����������ˮ����֪298 Kʱ�������ʵ�Ksp=5.6�� 10-12������ʱ�����ҺpH= 13. 00������¶��²�������Һ�е�M�������ʵ���Ũ��Ϊ___________mol/L�� (4)��ѧ��Ӧ3W2(g)+Y2(g)  2YW3(g) ��H<0������Ӧ�ﵽƽ��ʱ���ϸı����������ı�Y2��W2��YW3����������ͼ��ʾ��Ӧ�����뷴Ӧ���̵Ĺ�ϵ�����б�ʾƽ��������YW3�ĺ�����ߵ�һ��ʱ����____________�� 2YW3(g) ��H<0������Ӧ�ﵽƽ��ʱ���ϸı����������ı�Y2��W2��YW3����������ͼ��ʾ��Ӧ�����뷴Ӧ���̵Ĺ�ϵ�����б�ʾƽ��������YW3�ĺ�����ߵ�һ��ʱ����____________�� |
 |
(1)��������IA�壻2Mg+CO2 2MgO+C
2MgO+C
(2)���Ӽ������ۼ���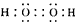
(3)5. 6��10-10
(4)t0��t1
(2)���Ӽ������ۼ���
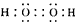
(3)5. 6��10-10
(4)t0��t1

��ϰ��ϵ�д�
�����Ŀ
�±��Dz��ֶ�����Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼۣ����ݱ��е���Ϣ���ж�����������ȷ���ǣ�������
|
�±��Dz��ֶ�����Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼۣ����ݱ�����Ϣ���ж�����������ȷ���ǣ�������
|
