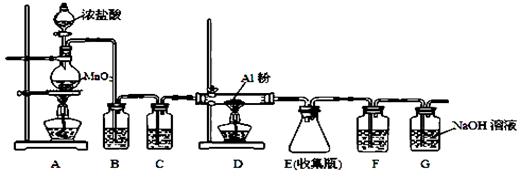
��Ŀ����
�ڳ����ķǽ������仯�����У�
��1�������ĵ����Խ��ڵ���;�Ե��֮�䣬�����õ� ���ϣ�������ˮ��Һ�׳�Ϊ �����Ʊ��轺��ľ�ķ������ԭ�ϣ��մɡ� ��ˮ��ȹ����β�Ʒ��ʹ�����������ǽ������ϡ�
��2����Ԫ������Ҫ�ġ�����Ԫ�ء���������ͨ��NaOH��Һ�п��Եõ�Ư��Һ����Ӧ�����ӷ���ʽ�� ��Ư��Һ��Ч�ɷֵĻ�ѧʽΪ ��
��3��ˮ�dz����ķǽ���Ԫ�ػ�����밴Ҫ��д������ˮ�μӵķ�Ӧ�Ļ�ѧ����ʽ��
��ˮֻ����������
��NO2����ͨ��ˮ�У�
��ˮ����������������ԭ����
��1���뵼�� ˮ���� ����
��2��Cl2+2OH��=Cl��+ClO��+H2O NaClO
��3����2Na+2H2O 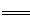 2NaOH+H2�� ��
2NaOH+H2�� ��
��3NO2+H2O  2HNO3+NO
2HNO3+NO
��2H2O  2H2��+O2��
2H2��+O2��
���������������1�������ĵ����Խ��ڵ���;�Ե��֮�䣬�����õİ뵼����ϣ�������ˮ��Һ��
��Ϊˮ�������մɡ�������ˮ����ʹ�����������ǽ������ϣ�
��2������������������Һ��Ӧ�����Ȼ��ơ��������ơ�ˮ�����ӷ���ʽΪCl2+2OH��=Cl��+ClO��+H2O������Ư��Һ��Ч�ɷ��Ǵ������ƣ���ѧʽΪNaClO��
��3����ˮֻ��������������ˮ�ķ�Ӧ������ˮ�����ķ�Ӧ��̼��ˮ�����ķ�Ӧ�ȣ�
��NO2����ͨ��ˮ�����������һ����������ѧ����ʽΪ3NO2+H2O  2HNO3+NO��
2HNO3+NO��
��ˮ����������������ԭ����ˮ�ĵ�⣬2H2O  2H2��+O2��
2H2��+O2��
���㣺�������ʵ��׳ơ����ǽ������ϡ������;���жϣ�Ư�۵����ɣ���ѧ����ʽ����д

���������ʡ���������ƵƵ������Ϊ��ֹ�ڴ���֮���߲����У�������Ҫ�����ĸ�����������Ư���ȡ�
(1)����������Ŀǰ�����Ϲ��ϵĵ��Ĵ���Ч�����Ĺ�����������������KClO3��SO2��H2SO4�����·�Ӧ�Ƶá���д����Ӧ�����ӷ���ʽ�� ��
(2)��̼������һ������̬Ư������ѧʽ�ɱ�ʾΪNa2CO3��3H2O2��������Na2CO3��H2O2��˫�����ʡ���̼�������������ʾ��ᷢ����ѧ��Ӧ��ʧЧ�����й�̼����ֻ������������Ӧ���� ��
| A��MnO2 | B��KMnO4��Һ | C��ϡ���� | D��Na2SO3��Һ |
��ҵ����̼���̿�Ϊ��Ҫԭ������MnO2�Ĺ����������£�
�й��������↑ʼ�����ͳ�����ȫ��pH���±���
| �������� | Al��OH��3 | Fe��OH��3 | Fe��OH��2 | Cu��OH��2 | Pb��OH��2 | Mn��OH��2 |
| ��ʼ������pH | 3.3 | 1.5 | 6.5 | 4.2 | 8.0 | 8.3 |
| ������ȫ��pH | 5.2 | 3.7 | 9.7 | 6.7 | 8.8 | 9.8 |
��ش��������⣺
��1�����ǰ��̼���̿�����������_________________________________��
��2����������Һ�к���Mn2����SO42-������������Fe2����Fe3����Al3����Cu2����Pb2���ȣ�����ӹ������£�
�ټ���MnO2��Fe2���������䷴Ӧ�����ӷ���ʽΪ___________________________________��
�ڼ���CaO����Һ��pH����5.2��6.0������ҪĿ����_____________________________��
�ۼ���BaS����ȥCu2����Pb2�����ټ���NaF��Һ����ȥ________��
��3������ҺA�л��յ���Ҫ������________�������ʳ��������ʡ�
��4��MnO2��Ʒ�к�������Mn3O4��������ϡ���ᴦ��������ת��ΪMnSO4��MnO2��Ȼ��������������Mn2��ת��ΪMnO2���Ƶ�����MnO2��д��Mn3O4��ϡ���ᷴӦ�Ļ�ѧ����ʽ��______________________________________��

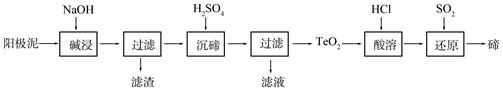
 CO(NH2)2 (l��+ H2O (l)��
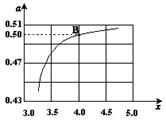
CO(NH2)2 (l��+ H2O (l)��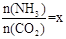 ����ͼ�ǰ�̼�ȣ�x����CO2ƽ��ת���ʣ������Ĺ�ϵ��������x����������ԭ���� ��
����ͼ�ǰ�̼�ȣ�x����CO2ƽ��ת���ʣ������Ĺ�ϵ��������x����������ԭ���� ��