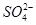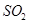��Ŀ����
���к�������NaCl��Na2SO4��Na2CO3�����ʵ�NaNO3��Һ��ѡ���ʵ����Լ���ȥ���ʣ��õ�������NaNO3���壬ʵ����������ͼ��ʾ��
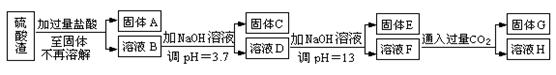
��1������A����Ҫ�ɷ��� �� ���ѧʽ����
��2�����з�Ӧ�����ӷ���ʽ�� ��
��3���٢ڢ��о����еķ�������� ��
��4�����м��������Na2CO3��Һ��Ŀ���� ��
��5����Һ3�����������Եõ�NaNO3���壬��Һ3�п϶����е������� ��Ϊ�˳�ȥ���ʣ�������Һ3�м��������� ��
��6��ʵ����������ʵ���õ�NaNO3��������500 mL 0.40 mol/L NaNO3��Һ��
��������Һʱ���������²�����a�����ݣ�b�����㣻c���ܽ⣻d��ҡ�ȣ�e��ת�ƣ�f��ϴ�ӣ�j����������ȡNaNO3����������� g�����ղ���˳��4���� ������ţ���
��ijͬѧת����Һ�IJ�����ͼ��ʾ��ͼ���������������ձ��� ����ͬѧ�����еĴ����� ��


��1������A����Ҫ�ɷ��� �� ���ѧʽ����
��2�����з�Ӧ�����ӷ���ʽ�� ��
��3���٢ڢ��о����еķ�������� ��
��4�����м��������Na2CO3��Һ��Ŀ���� ��
��5����Һ3�����������Եõ�NaNO3���壬��Һ3�п϶����е������� ��Ϊ�˳�ȥ���ʣ�������Һ3�м��������� ��
��6��ʵ����������ʵ���õ�NaNO3��������500 mL 0.40 mol/L NaNO3��Һ��
��������Һʱ���������²�����a�����ݣ�b�����㣻c���ܽ⣻d��ҡ�ȣ�e��ת�ƣ�f��ϴ�ӣ�j����������ȡNaNO3����������� g�����ղ���˳��4���� ������ţ���
��ijͬѧת����Һ�IJ�����ͼ��ʾ��ͼ���������������ձ��� ����ͬѧ�����еĴ����� ��

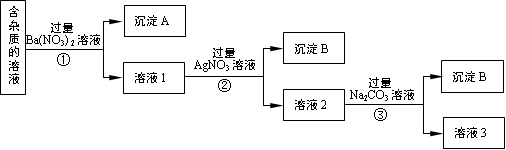
��1��BaSO4 BaCO3
��2��Ag++ Cl��="=" AgCl��
��3������
��4����ȥBa2+��Ag+
��5��Na2CO3��CO32�� ϡ����
��6����17.0 e ��500mL����ƿ δ�ò���������
��2��Ag++ Cl��="=" AgCl��
��3������
��4����ȥBa2+��Ag+
��5��Na2CO3��CO32�� ϡ����
��6����17.0 e ��500mL����ƿ δ�ò���������
�����������1���������ʵ�NaNO3��Һ��Һ�м��������Ba(NO3)2,������Ӧ��Na2SO4+ Ba(NO3)2= BaSO4��+ 2NaNO3��Na2CO3+ Ba(NO3)2= BaCO3��+ 2NaNO3����A����Ҫ�ɷ���BaSO4��BaCO3����2����Һ1�к���NaCl��NaNO3��������Ba(NO3)2�������м��������Ag NO3��Һ��������Ӧ��NaCl +Ag NO3= AgCl��+ NaNO3�����ӷ���ʽΪ��Ag++ Cl��="=" AgCl������3���٢ڢ��ж��Ƿ��������Թ�����Һ��IJ����������еķ�������ǹ��ˡ���4������Һ2�м��������Na2CO3��Һ��������Ӧ��Ba(NO3)2+ Na2CO3= BaCO3��+ 2NaNO3��2AgNO3+ Na2CO3= Ag2CO3��+ 2NaNO3.���Ԣ��м��������Na2CO3��Һ��Ŀ���dz�ȥBa2+��Ag+����5�����ڢۼ��������Na2CO3��Һ������3�����������Եõ�NaNO3���壬��Һ3�п϶����е�������Na2CO3��Ϊ�˳�ȥ���ʣ�������Һ3�м���������ϡ���ᡣ��6����������Һʱ����ȡNaNO3�����������0.5L ��0.40 mol/L��85g/mol=17.0g.���ղ���˳��4����e��ת��.��ijͬѧת����Һ�IJ�������ͼ��ʾ��ͼ���������������ձ���500mL����ƿ;��ͬѧ�����еĴ�����δ�ò�����������

��ϰ��ϵ�д�
 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д�
�����Ŀ