��Ŀ����
��1��������ijǿ����ҺpH��a��ǿ����ҺpH��b����֪a��b��12�������Һ���pH��7��������Һ���V(��)�ͼ���Һ���V(��)�ı�ֵ��ϵΪ ��
��2������Ũ�Ⱦ�Ϊ0.1 mol/L��������Һ���������������ڴ�����������ƣ������ᣬ������Һ����ˮ�������OH��Ũ���ɴ�С��˳���ǣ�����ţ� ��
��3����֪100�� KW��10��12���������¶���pH��8��Ba(OH)2��Һ��pH��5��ϡ�����ϣ�������100��ĺ��£���ʹ�����ҺpH��7����Ba(OH)2��Һ����������֮��Ϊ ��
��2������Ũ�Ⱦ�Ϊ0.1 mol/L��������Һ���������������ڴ�����������ƣ������ᣬ������Һ����ˮ�������OH��Ũ���ɴ�С��˳���ǣ�����ţ� ��
��3����֪100�� KW��10��12���������¶���pH��8��Ba(OH)2��Һ��pH��5��ϡ�����ϣ�������100��ĺ��£���ʹ�����ҺpH��7����Ba(OH)2��Һ����������֮��Ϊ ��
��1�� V(��):V(��)=1:100 ��2�� ��>�ۣ���>�� ��3��2:9
�����������1��������ijǿ����ҺpH��a������Һ��������Ũ����10��amol/L��ǿ����ҺpH��b������Һ��OH��Ũ����10b��14mol/L����֪�����Һ���pH��7����V(��)��10��amol/L��V(��)��10b��14mol/L������Ϊa��b��12������V(��):V(��)=10a��b��14��10��2��1:100��
��2�����������Ƕ�Ԫǿ�����Һ��OH��Ũ����0.2mol/L�����������ᣬ���ڵ���ƽ�⣬����Һ��������Ũ����С��0.1mol/L������������һԪǿ�����Һ��OH��Ũ����0.1mol/L��������һԪǿ�ᣬ����Һ��������Ũ����0.1mol/L��һԪ�������������ӻ��������OH������ˮ�ĵ��룬�������ӻ�OH��Ũ��Խ�����Ƴ̶�Խ�����������Һ����ˮ�������OH��Ũ���ɴ�С��˳���Ǣ�>�ۣ���>�١�
��3����֪100�� KW��10��12������¶���pH��8��Ba(OH)2��Һ��OH��Ũ����10��4mol/L��pH��5��ϡ������Һ��������Ũ����10��5mol/L�������Ϻ���Һ��pH��7����˵�����ǹ����ģ�������
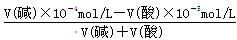 ��10��5mol/L�����V(��):V(��)=2:9��
��10��5mol/L�����V(��):V(��)=2:9��
��ϰ��ϵ�д�
�����Ŀ
 H++OH? ����ش�
H++OH? ����ش�
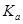 =____________mol/L��
=____________mol/L��