��Ŀ����
����ƽ����ϵ2SO2��g��+O2��g�� 2SO3��g�� ��H<0�����н�������ȷ���ǣ� ��
2SO3��g�� ��H<0�����н�������ȷ���ǣ� ��
 2SO3��g�� ��H<0�����н�������ȷ���ǣ� ��
2SO3��g�� ��H<0�����н�������ȷ���ǣ� ��| A�����¶Ȳ��䣬���������������һ������ʱ��SO2Ũ�ȱ�Ϊԭ����0.5�� |
| B����ƽ��ʱSO2��O2��ת������ȣ�˵����Ӧ��ʼʱ�����ߵ����ʵ������Ϊ2��1 |
| C������ƽ����ϵ�з����SO2�������������SO2��ת���ʺͼӿ�����Ӧ���� |
| D��ƽ��״̬ʱSO2��O2��SO3�����ʵ���֮��һ��Ϊ2��1��2 |
B
��

��ϰ��ϵ�д�
�����Ŀ
 P(g)��Q(g) ��H��0��
P(g)��Q(g) ��H��0�� 
 CH3OH(g)+H2O(g) ��H=��49.0 kJ/mol�� ��
CH3OH(g)+H2O(g) ��H=��49.0 kJ/mol�� ��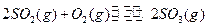
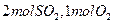 ����ƽ��ʱ����
����ƽ��ʱ����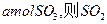 ��ת����Ϊ ��
��ת����Ϊ ��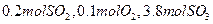 ����ƽ��ʱSO2�����ʵ���Ϊ mol��
����ƽ��ʱSO2�����ʵ���Ϊ mol�� CO2��g��+H2��g�����仯ѧƽ�ⳣ��K���¶�k�Ĺ�ϵ����
CO2��g��+H2��g�����仯ѧƽ�ⳣ��K���¶�k�Ĺ�ϵ���� ����
����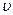 ����H2��=
����H2��= 2NO2(g) ��H��0��
2NO2(g) ��H��0�� Z+W���������������������£�����ѹǿ����Ӧ���ʱ仯����ͼ��ʾ
Z+W���������������������£�����ѹǿ����Ӧ���ʱ仯����ͼ��ʾ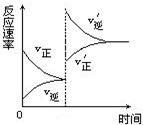 ����ͼ���й���X��Y��Z��W�������ʵľۼ�״̬Ϊ:
����ͼ���й���X��Y��Z��W�������ʵľۼ�״̬Ϊ:
 ������5
������5 ����D��Ũ��Ϊ
����D��Ũ��Ϊ ��C�ķ�Ӧ����Ϊ
��C�ķ�Ӧ����Ϊ ������˵���в���ȷ���ǣ� ��
������˵���в���ȷ���ǣ� ��

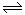 3C(g)��ֻ�з�Ӧ����ƽ��ʱ�ž��е�������
3C(g)��ֻ�з�Ӧ����ƽ��ʱ�ž��е�������