��Ŀ����
����Ŀ�����������������ڵ�����Ԫ��A��B��C��D�����ǵ�ԭ�Ӱ뾶���α�С��A���Ӻ�B���ӵĵ��Ӳ�������㣬�����γ�BA2�͵����ӻ����C�����Ӵ�3��������Һ�������Ų�������ͬ��D��̬�⻯��ķ���ʽΪH2D��D����������������е�����������40����Dԭ�Ӻ�����16�����ӡ�
�Իش�
(1)��Ҫ����գ�
AԪ�ص�����______________��BԪ�صķ���____________��
Cԭ�ӵ���Χ�����Ų�ʽΪ____________��D��ԭ�ӽṹʾ��ͼ____________��
(2)B��C��D�ĵ�һ�������ɴ�С��˳���ǣ�______________(��Ԫ�ط���)��
(3)A��B��C�ĵ縺���ɴ�С��˳���ǣ�____________(��Ԫ�ط���)��
(4)A��B�γɵĻ�����ĵ���ʽ__________________��
(5)��CͶ�뵽��KOH��Һ������Ϊ______________����Ӧ�����ӷ�Ӧ����ʽΪ________________________ ��
���𰸡��� Mg 3s23p1  S��Mg��Al Br��Al��Mg
S��Mg��Al Br��Al��Mg ![]() �����ܽ⣬����ų���ɫ��ζ������ 2Al + 2OH- +2H2O=2AlO2- + 3H2��
�����ܽ⣬����ų���ɫ��ζ������ 2Al + 2OH- +2H2O=2AlO2- + 3H2��
��������
���������������ڵ�����Ԫ��A��B��C��D�����ǵ�ԭ�Ӱ뾶���α�С��C�����Ӵ�3����λ������Һ�������Ų�������ͬ��CΪAlԪ�أ�D����̬�⻯��ͨʽΪH2D��D�����������Ļ�ѧʽΪDO3��![]() ��100%=40%�����Ar��D��=32��Dԭ�Ӻ�����16�����ӣ���D��������Ϊ32-16=16��DΪSԪ�أ�A���Ӻ�B���ӵĵ��Ӳ����2�㣬�����γ�BA2�͵����ӻ����BΪ+2�۽���Ԫ�أ�AΪ-1�۷ǽ���Ԫ�أ����������������ڵ�����Ԫ��A��B��C��D�����ǵ�ԭ�Ӱ뾶���α�С����Aֻ��ΪBr��BΪMg���ݴ˷������
��100%=40%�����Ar��D��=32��Dԭ�Ӻ�����16�����ӣ���D��������Ϊ32-16=16��DΪSԪ�أ�A���Ӻ�B���ӵĵ��Ӳ����2�㣬�����γ�BA2�͵����ӻ����BΪ+2�۽���Ԫ�أ�AΪ-1�۷ǽ���Ԫ�أ����������������ڵ�����Ԫ��A��B��C��D�����ǵ�ԭ�Ӱ뾶���α�С����Aֻ��ΪBr��BΪMg���ݴ˷������
��������������AΪBrԪ�أ�BΪMgԪ�أ�CΪAlԪ�أ�DΪSԪ�ء�
(1)A��B��C��D�ֱ�ΪBr��Mg��Al��S��AԪ�ص�����Ϊ�壻BԪ�صķ���ΪMg��Cԭ�ӵ���Χ�����Ų�ʽΪ3s23p1��D��ԭ�ӽṹʾ��ͼΪ ���ʴ�Ϊ���壻Mg�� 3s23p1��
���ʴ�Ϊ���壻Mg�� 3s23p1�� ��
��
(2)ͬ����Ԫ�أ������ң�Ԫ�صĵ�һ�����ܳ��������ƣ�����IIA�塢��VA��Ԫ�صĵ�һ�����ܴ�������Ԫ�ء���B��C��D�ĵ�һ�������ɴ�С��˳��ΪS��Mg��Al���ʴ�Ϊ��S��Mg��Al��
(3)ͬ���ڣ�������ҵ縺������ͬ�������϶��µ縺�Խ��ͣ��ǽ�����Խǿ�縺��Խǿ����A��B��C�ĵ縺���ɴ�С��˳��ΪBr��Al��Mg���ʴ�Ϊ��Br��Al��Mg��
(4)A��B�γɵĻ�����Ϊ�廯þ���������ӻ��������ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(5)����Ͷ�뵽��KOH��Һ�У�������������Ѹ�ٷ�Ӧ�ų���������Ӧ�����ӷ���ʽΪ2Al + 2OH- +2H2O=2AlO2- + 3H2�����ʴ�Ϊ�������ܽ⣬����ų���ɫ��ζ�����壻2Al + 2OH- +2H2O=2AlO2- + 3H2����

 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д�����Ŀ������ʵ����������ͻ���۾���ȷ����
ѡ�� | ʵ��Ŀ�� | ���� | ���ۻ���� |
A | ���� | ȡ����Һ��������������� | Һ��ֲ㣬���������ữ�� |
B | ����ij��Һ������ | ȡ����Һ�������������ᣬ������ų���������ͨ�����ʯ��ˮ�� | ����ʯ��ˮ����ǣ��� |
C | ������Һ�е� | ȡ����Һ��������ͨ���������ټ�KSCN��Һ | ��Һ���ɫ���� |
D | ����ʳ�����Ƿ� | ȡ����ʳ������ˮ������������ | ��Һ����ɫ���� |
A.AB.BC.CD.D
����Ŀ��ȼú�������к��� SO2��Ϊ�����������������������ö��ַ���ʵ����������
��.(1)��ʪʽ���շ����������ռ��� SO2 ������Ӧ�Ӷ����������Լ����ʺ������÷����ռ�����_____(����ĸ���)��
a. ʯ���� b.CaCl2��Һ
(2)ij�������ú� SO2 ������������Cr2O72-�����Է�ˮ���������з�Ӧ��ĸ�Ԫ����Cr3+��ʽ���ڣ������������£�
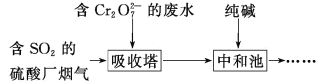
���� SO2 ����������ˮʱ�������� SO2 ��_____�ԡ�
���������з�����Ӧ�����ӷ���ʽΪ_____��
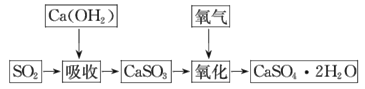
��.ʯ��-ʯ�෨���ռ�dz��õ���������ʯ��-ʯ�෨�����շ�ӦΪCa(OH)2+SO2= CaSO3��+H2O�����ղ�����������ɹܵ���������������������ӦΪ2CaSO3+O2+4H2O =2CaSO4��2H2O����������ͼ��
�ռ�����շ�ӦΪ2NaOH+SO2=Na2SO3+H2O���÷����ص����������Ƽ���ǿ�����տ졢Ч�ʸߡ���������ͼ��

��֪��
�Լ� | Ca(OH)2 | NaOH |
�۸�(Ԫ/kg) | 0.36 | 2.9 |
���� SO2 �ijɱ�(Ԫ/mol) | 0.027 | 0.232 |
(3)ʯ��-ʯ�෨���ռ��ȣ�ʯ��-ʯ�෨���ŵ���_______��ȱ����_______��
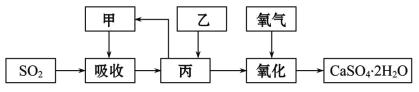
(4)ijѧϰС����ʯ��-ʯ�෨���ռ�Ļ����ϣ����һ���Ľ��ġ���ʵ������ѭ������������������ͼ�еļס��ҡ�������_____��_____��_____(�ѧʽ)