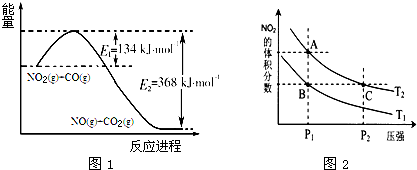
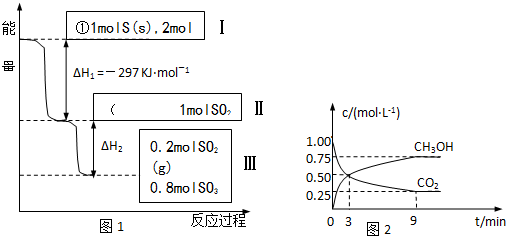
��Ŀ����
����˵�����ʾ������ȷ���ǣ�������
| A�������ʵ������������������ֱ���ȫȼ�գ����߷ų������� | B����H+��aq��+OH-��aq���TH2O��l����H=-57.3kJ?mol-1��֪��������1 mol CH3COOH��ϡ��Һ�뺬1 mol NaOH��ϡ��Һ��ϣ��ų�������С��57.3 kJ | C��300�桢30MPa�£���0.5molN2��g����1.5mol H2��g�������ܱ������г�ַ�Ӧ����NH3��g��������19.3kJ�����Ȼ�ѧ����ʽΪ��N2��g��+3H2��g��=2NH3��g����H=-38.6kJ?mol-1 | D����C��ʯī���TC�����ʯ����H=+1.90 kJ?mol-1��֪�����ʯ��ʯī�ȶ� |
������A���������仯Ϊ�����Ϊ���ȹ��̣�
B��CH3COOHΪ���ᣬ����ʱ���ȣ�
C���ϳɰ���ӦΪ���淴Ӧ��
D�����ݷ�Ӧ�����仯�ж�����������С����������Խ��ԽԽ���ã�
B��CH3COOHΪ���ᣬ����ʱ���ȣ�
C���ϳɰ���ӦΪ���淴Ӧ��
D�����ݷ�Ӧ�����仯�ж�����������С����������Խ��ԽԽ���ã�
����⣺A���������仯Ϊ�����Ϊ���ȹ��̣����������������������������зֱ���ȫȼ�գ��ų������������࣬��A����
B������Ϊ������ʣ��������ȣ����Խ���1 mol CH3COOH��ϡ��Һ�뺬1 mol NaOH��ϡ��Һ��ϣ��ų�������С��57.3kJ����B��ȷ��
C�����ںϳɰ���ӦΪ���淴Ӧ����Ӧ�ﲻ��ȫ��ת��Ϊ���������0.5molN2��g����1.5mol H2��g����ȫ��Ӧ�ų�����������19.3kJ����C����
D����C��ʯī����C�����ʯ������H=+19kJ/mol����Ӧ���ȣ����ʯ��������ʯī����֪ʯī�Ƚ��ʯ�ȶ�����D����
��ѡB��
B������Ϊ������ʣ��������ȣ����Խ���1 mol CH3COOH��ϡ��Һ�뺬1 mol NaOH��ϡ��Һ��ϣ��ų�������С��57.3kJ����B��ȷ��
C�����ںϳɰ���ӦΪ���淴Ӧ����Ӧ�ﲻ��ȫ��ת��Ϊ���������0.5molN2��g����1.5mol H2��g����ȫ��Ӧ�ų�����������19.3kJ����C����
D����C��ʯī����C�����ʯ������H=+19kJ/mol����Ӧ���ȣ����ʯ��������ʯī����֪ʯī�Ƚ��ʯ�ȶ�����D����
��ѡB��
���������⿼���˷�Ӧ�����仯���Ȼ�ѧ����ʽ�������жϣ������������ȶ��ԵıȽϷ�������Ŀ�ѶȲ���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
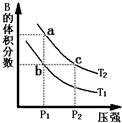 ���ڷ�ӦA��g��?2B��g����H��0�����¶�ΪT1��T2ʱ��ƽ����ϵ��B�����������ѹǿ�仯��������ͼ��ʾ���ش����и��⣮
���ڷ�ӦA��g��?2B��g����H��0�����¶�ΪT1��T2ʱ��ƽ����ϵ��B�����������ѹǿ�仯��������ͼ��ʾ���ش����и��⣮