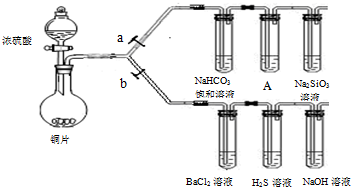
��Ŀ����
����Ҫ��������и�С��ʵ��Ŀ�ġ���a��bΪ���ɼУ����ȼ��̶�װ������ȥ��

(1)��֤̼����ǽ����Ե����ǿ��������֪���ԣ������� >̼�ᣩ
�ٽ�������_________________����ҩƷ��a�ر� b��Ȼ�����Ũ���ᣬ���ȡ�
��ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽ�ǣ� ��
װ��A�е��Լ������ǣ� ��
����˵��̼�ķǽ����Աȹ�ǿ��ʵ�������ǣ� ��
(2)��֤ SO2�������ԡ���ԭ�Ժ������������ͨ�ԡ�
�ٴ�b���ر�a��
��H2S��Һ����dz��ɫ���dz��֣���ѧ����ʽ�ǣ�
��BaCl2��Һ��������������ֳ����ݣ��քe�μ�������Һ���������ij����Ļ�ѧʽ�����±���Ӧλ�ã�

д��SO2����ˮ��Ӧ�����ӷ���ʽ ��
(1) �ټ��װ�������ԣ�2�֣� ��Cu + 2H2SO4(Ũ)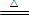 CuSO4 + SO2��+
2H2O��2�֣�
CuSO4 + SO2��+
2H2O��2�֣�
����KMnO4��Һ����ˮ��NaHCO3��Һ��2�֣� ��Na2SiO3��Һ�в�����״������2�֣�
(2) ��2H2S + SO2 = 3S��+ 2H2O��2�֣� ��BaSO4��2�֣� BaSO3��2�֣�
Cl2 + SO2 + 2H2O = SO42��+ 4H��+ 2Cl����2�֣�
����������1��װ��ͼ��װ���Ժ�����Ҫ����װ�õ������ԡ�Ũ�������ǿ�����ԣ��ڼ��ȵ��������ܰ�ͭ������������ͭ��SO2��ˮ�����������������ǿ��̼��ģ��������ɵ�SO2ͨ�뵽̼��������Һ�У�������CO2���塣��̼���������ǿ�ڹ���ģ�����CO2ͨ�뵽��������Һ�У����������ᾧ�塣Ϊ�˷�ֹSO2�Ĵ��ڸ���CO2�����Ƶķ�Ӧ����Ҫ��ͨ���������Һ֮ǰ���Ȱ�SO2��ȥ������ѡ�����Ը��������Һ����ˮ��̼��������Һ�ȡ�
��2��H2S�е���Ԫ�ش�����ͼۣ�SO2�ܰ������������ɵ�����ӦʽΪ2H2S + SO2 = 3S��+ 2H2O����������ǿ�����ԣ��ܰ�SO2�����������ᣬ�Ӷ��������ᱵ��������ˮ�������ᷴӦ����������泥��������������ᱵ������