��Ŀ����
�ֱ�ȡ40mL��0.50mol/L������40mL 0.55mol/L����������Һ�����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ���ش��������⡣
��1��������ϡǿ�ᡢϡǿ�Ӧ����1molˮʱ�ų�57.3kJ��������д����ʾϡ�����ϡ����������Һ��Ӧ���к��ȵ��Ȼ�ѧ����ʽ ��
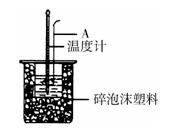
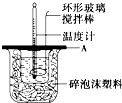
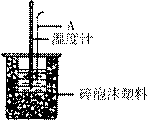 ��2������ͼ��ʾ������A��������_______________����ʵ������У���������¶ȼ��ϵ�����ˮ��ϴ�ɾ�ֱ�Ӳ���NaOH��Һ���¶ȣ����õġ�H ���ƫ����ƫС������Ӱ�족����
��2������ͼ��ʾ������A��������_______________����ʵ������У���������¶ȼ��ϵ�����ˮ��ϴ�ɾ�ֱ�Ӳ���NaOH��Һ���¶ȣ����õġ�H ���ƫ����ƫС������Ӱ�족����
��3���������������������Һ���ܶȶ���1g/cm3����֪�кͺ�������Һ�ı�����c��4.18J/��g?�棩��Ϊ�˼����к��ȣ�ijѧ��ʵ���¼�������£�
ʵ����� | ��ʼ�¶�t1�� | ��ֹ�¶�t2�� | |
���� | �������� | �����Һ | |
1 | 20��0 | 20��1 | 23��2 |
2 | 20��2 | 20��4 | 23��4 |
3 | 20��5 | 20��6 | 23��6 |
���ݸ�ѧ����ʵ�����ݼ��㣬��ʵ���õ��к��ȡ�H��________________�����������һλС����
��4�� ����ܡ����ܡ�����Ba(OH)2������������������������Һ�������� ��
��1��1/2H2SO4(aq)+ 1/2 Ba(OH)2(aq)== 1/2BaSO4(s)+H2O(1)����H=-57.3kJ��mol
H+(aq)+OH-(aq)=H2O(1)����H = ��57.3kJ?mol-1��
��2�����β�������� ƫ��
��3��-51.8 kJ��mol
��4������ H2SO4��Ba(OH)2��Ӧ���ɵ�BaSO4�����������Ȼ�Ӱ�췴Ӧ�ķ�Ӧ��
�������:�Ȼ�ѧ����ʽ��д����ȷ���е�ͬѧ©�������ʵľۼ�״̬����Ӧ�Ⱥ���û��λ�����е�д����A�����ơ�����������ȷ�ȡ���Щ����ĵ����Ǻ����˿α�ʵ��Ļعˡ��������һ��Ҫ���α��úù�һ�顣
����ָ��:1. �Ȼ�ѧ����ʽ����д����
2. �к��Ȳⶨʵ����������ģ�ʵ�鲽�衢���ܻ�������ķ�����
3. �ع�α�

��7�֣��ֱ�ȡ40mL��0.50 mol/L������40mL��0.55 mol/L����������Һ�����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ���ش��������⡣
(1)������ϡǿ�ᡢϡǿ�Ӧ����1 mol ˮʱ�ų�57.3 kJ��������д����ʾϡ�����ϡ����������Һ��Ӧ���к��ȵ��Ȼ�ѧ����ʽ__________________________________��
(2)��ͼ��ʾ��AΪ��ĭ���ϰ壬����������С�ף��ֱ�����¶ȼƺͻ��β��������������С�ײ��ܿ��ù�����ԭ����_____________________________________________��
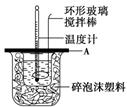
(3)�������������������Һ���ܶȶ���1 g/cm3����֪�кͺ�������Һ�ı�����c��4.18 J/(g����)��Ϊ�˼����к��ȣ�ʵ��ʱ���������������(�����)________��
A����Ӧǰ������Һ���¶�
B����Ӧǰ������Һ������
C����Ӧǰ����������Һ���¶�
D����Ӧǰ����������Һ������
E����Ӧ������Һ������¶�
F����Ӧ������Һ������
(4)ijѧ��ʵ���¼�������£�
|
ʵ�� ��� |
��ʼ�¶�t1/�� |
��ֹ�¶�t2/�� |
|
|
���� |
�������� |
�����Һ |
|
|
1 |
20.0 |
20.1 |
23.2 |
|
2 |
20.2 |
20.4 |
23.4 |
|
3 |
20.5 |
20.6 |
23.6 |
���ݸ�ѧ����ʵ�����ݼ��㣬��ʵ���õ��к��Ȧ�H��________��

 �ֱ�ȡ40mL��0.50mol/L������40mL��0.55mol/L����������Һ�����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ���ش��������⣮
�ֱ�ȡ40mL��0.50mol/L������40mL��0.55mol/L����������Һ�����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ���ش��������⣮