��Ŀ����
2���ϳɾ����������オ���Ե��л��߷��Ӳ������л���ѧ�о�����Ҫ����֮һ���۴�����ϩ����PVAc��ˮ�����ɵľ���ϩ����PVA���������������オ���ԣ�������������ȫ�����в����PVB���йغϳ�·����ͼ�����ַ�Ӧ�����Ͳ�����ȥ����
��֪��
��
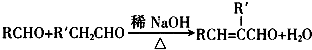 ��R��R���ʾ�������⣩
��R��R���ʾ�������⣩��
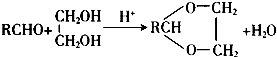
��ش�
��1��AΪ����һԪ������������������ԼΪ34.8%��A�Ļ�ѧ����Ϊ�Ҵ���PVA�Ľṹ��ʽΪ
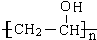 ��
����2��C�й����ŵ�������̼̼˫����ȩ����C������2-��ϩȩA��F�к˴Ź������׳���������C��D���������ţ���
��3����Ӧ�ٰ����ķ�Ӧ�����Ǽӳɷ�Ӧ����ȥ��Ӧ����Ӧ�ܵĻ�ѧ����ʽΪ$\frac{n}{2}$CH3CH2CH2CHO+
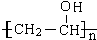 ��
��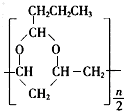 +$\frac{n}{2}$H2O��
+$\frac{n}{2}$H2O����4��PVAc����F�Ӿ۶��ɣ���F������ͬ�����ŵ�ͬ���칹�廹��4�֣�д������һ�ֵĽṹ��ʽ��HCOOCH=CHCH3��HCOOCH2CH=CH2��CH3OOCCH=CH2��HCOOC��CH3��=CH2������һ�֣���
���� AΪ����һԪ����ͨʽΪCnH2n+2O����������������ԼΪ34.8%������$\frac{16}{12n+2n+2+16}$��100%=34.8%�����n=2����AΪCH3CH2OH����PVAc�ṹ��֪��A��������EΪCH3COOH��E����Ȳ�����ӳɷ�Ӧ����FΪCH3COOCH=CH2��F�����Ӿ۷�Ӧ�õ�PVAc������ˮ��õ�PVAΪ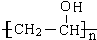 ��A��ͭ�������������������õ�BΪCH3CHO��B������Ϣ���еķ�Ӧ�õ�CΪCH3CH=CHCHO��C������ԭ��Ӧ����DΪCH3CH2CH2CHO��D��PVA������Ϣ���еķ�Ӧ��PVB���ݴ˴��⣮
��A��ͭ�������������������õ�BΪCH3CHO��B������Ϣ���еķ�Ӧ�õ�CΪCH3CH=CHCHO��C������ԭ��Ӧ����DΪCH3CH2CH2CHO��D��PVA������Ϣ���еķ�Ӧ��PVB���ݴ˴��⣮
��� �⣺AΪ����һԪ����ͨʽΪCnH2n+2O����������������ԼΪ34.8%������$\frac{16}{12n+2n+2+16}$��100%=34.8%�����n=2����AΪCH3CH2OH����PVAc�ṹ��֪��A��������EΪCH3COOH��E����Ȳ�����ӳɷ�Ӧ����FΪCH3COOCH=CH2��F�����Ӿ۷�Ӧ�õ�PVAc������ˮ��õ�PVAΪ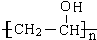 ��A��ͭ�������������������õ�BΪCH3CHO��B������Ϣ���еķ�Ӧ�õ�CΪCH3CH=CHCHO��C������ԭ��Ӧ����DΪCH3CH2CH2CHO��D��PVA������Ϣ���еķ�Ӧ��PVB��
��A��ͭ�������������������õ�BΪCH3CHO��B������Ϣ���еķ�Ӧ�õ�CΪCH3CH=CHCHO��C������ԭ��Ӧ����DΪCH3CH2CH2CHO��D��PVA������Ϣ���еķ�Ӧ��PVB��
��1��������������֪��A�Ļ�ѧ����Ϊ�Ҵ���PVA�Ľṹ��ʽΪ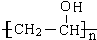 ��
��
�ʴ�Ϊ���Ҵ���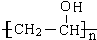 �� ��������
�� ��������
��2��CΪCH3CH=CHCHO��C�й����ŵ�������̼̼˫����ȩ����C������Ϊ2-��ϩȩ��A��F�к˴Ź������׳���������C��D������4�ַ壬
�ʴ�Ϊ��̼̼˫����ȩ����2-��ϩȩ��C��D��
��3����Ӧ������ȩ�ļӳɷ�Ӧ��Ȼ���ٷ���������ȥ��Ӧ��
��Ӧ�ܵĻ�ѧ����ʽΪ��$\frac{n}{2}$CH3CH2CH2CHO+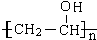 ��
�� +$\frac{n}{2}$H2O��
+$\frac{n}{2}$H2O��
�ʴ�Ϊ���ӳɷ�Ӧ����ȥ��Ӧ��
$\frac{n}{2}$CH3CH2CH2CHO+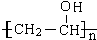 ��
�� +$\frac{n}{2}$H2O��
+$\frac{n}{2}$H2O��
��4��FΪCH3COOCH=CH2����F������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽΪ��HCOOCH=CHCH3��HCOOCH2CH=CH2��CH3OOCCH=CH2��HCOOC��CH3��=CH2��
�ʴ�Ϊ��4��HCOOCH=CHCH3��HCOOCH2CH=CH2��CH3OOCCH=CH2��HCOOC��CH3��=CH2������һ�֣���
���� ���⿼���л�����ƶ���ϳɣ��ؼ��Ǽ���ȷ��A�Ľṹ��ʽ���ٽ�Ϸ�Ӧ������PVAc��PVB�Ľṹ��ʽ�����ƶϣ��ۺϿ���ѧ�������������ۺ����û�ѧ֪ʶ��������ע������л�������ŵĽṹ�����ʣ���Ŀ�Ѷ��еȣ�

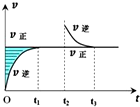
| A�� | �÷�Ӧ��Z��W-����Ϊ��̬ | |
| B�� | t1-t2��t3-t4��ʱ�Σ���Ӧ��ϵ������ƽ��Ħ��������������� | |
| C�� | ���¶��£����˷�Ӧ��ƽ�ⳣ������ʽΪK=[X]����t1-t2��t3-t4��ʱ�ε�c��X����ͬ | |
| D�� | ���ڸ��������£��÷�Ӧ�����Է����У���÷�Ӧ��ƽ�ⳣ��K���¶����߶����� |
| A�� | ��ˮ�е���ϡ���H++OH-�TH2O | |
| B�� | ����������Һ�е�������İ�ˮ��Al3++4OH-�TAlO2-+2H2O | |
| C�� | �Ͱ�ˮ��ͨ������CO2���壺NH3+H2O+CO2�TNH4++HCO3- | |
| D�� | ���Ȼ�淋�ϡ��Һ�е���������NaOH��Һ��NH4++OH-�TNH3•H2O |
| A�� | �������������ᣩ���ӱ���Na2CO3 ��Һ��������ú�Һ | |
| B�� | �Ҵ���ˮ��������������ʯ�ң����� | |
| C�� | ���ᣨ�Ҵ�������������ƣ����� | |
| D�� | ����Һ�壩������NaOH��Һ����Һ |
| A�� | ��ȥ���е������壺����NaOH��Һ�������÷ֲ��ȥˮ�� | |
| B�� | ��ȥ���������в������Ҵ������������Ũ���ᣬȻ����ȣ��ٷ�Һ | |
| C�� | �����ȵ�ͭ˿Ѹ�ٲ����Ҵ��У�������Σ��ɹ۲쵽ͭ˿�����ڣ������ŵ���ζ | |
| D�� | �������Һ�м�������ϡ���Ტ���ȣ�Ϊ�ⶨ���Ƿ���ˮ����Ƿ���ȫˮ�⣬��ʹ�õ����Լ��е�ˮ������Cu��OH��2����Һ |
| A�� | C6H14 | B�� | C7H16 | C�� | C8H18 | D�� | C9H20 |

 �����ķ���ʽ��C10H22
�����ķ���ʽ��C10H22